Description
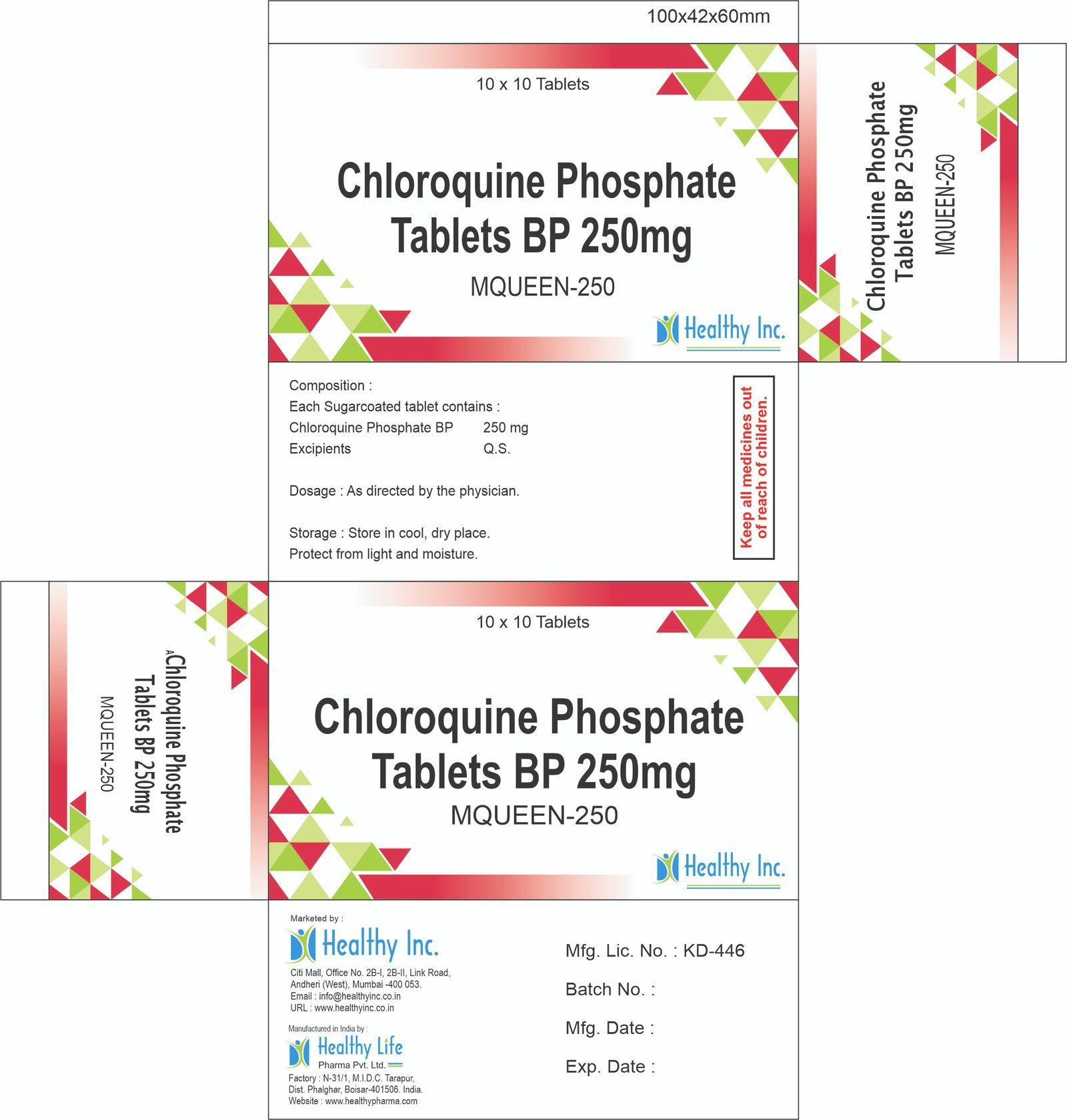
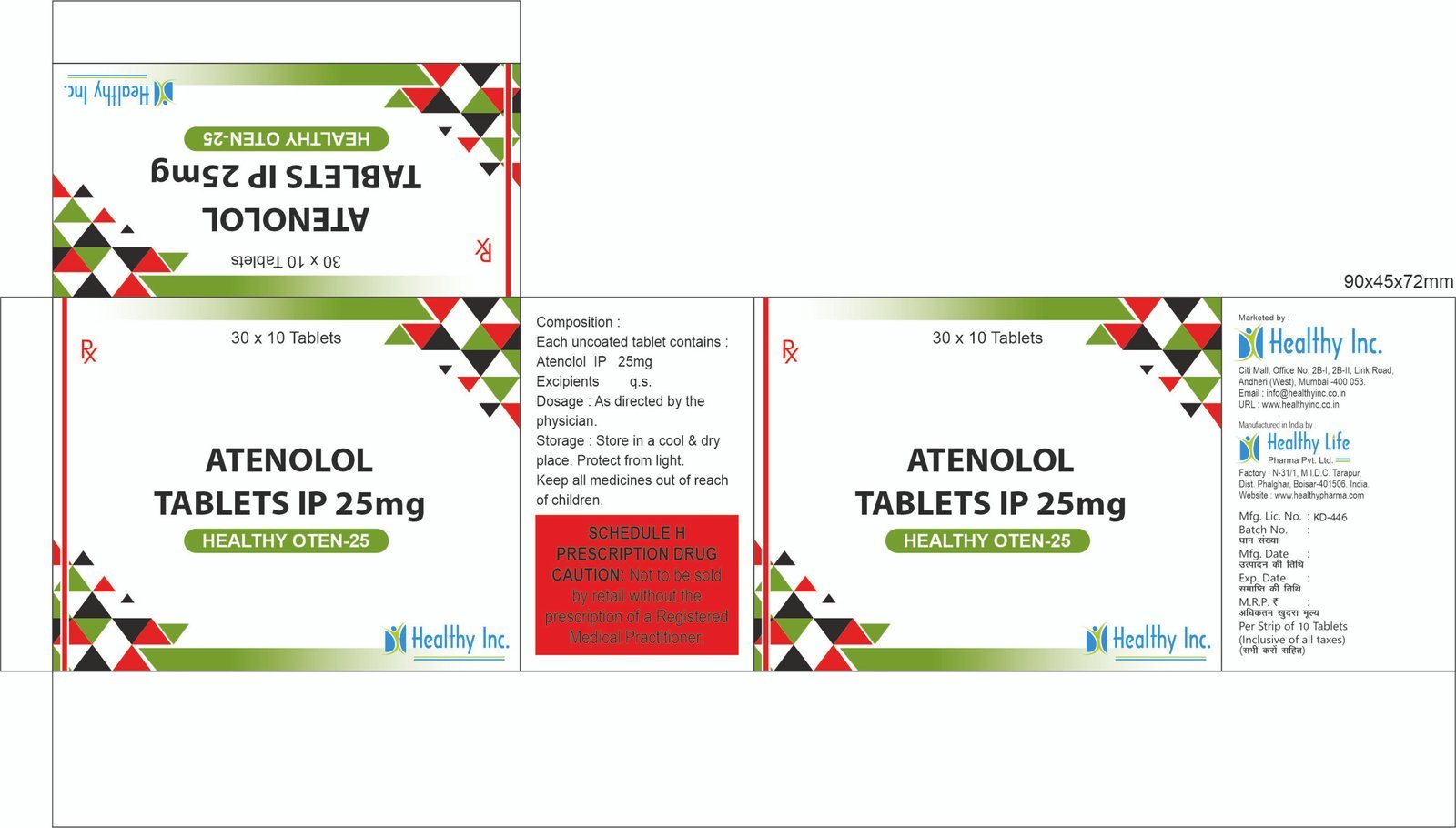
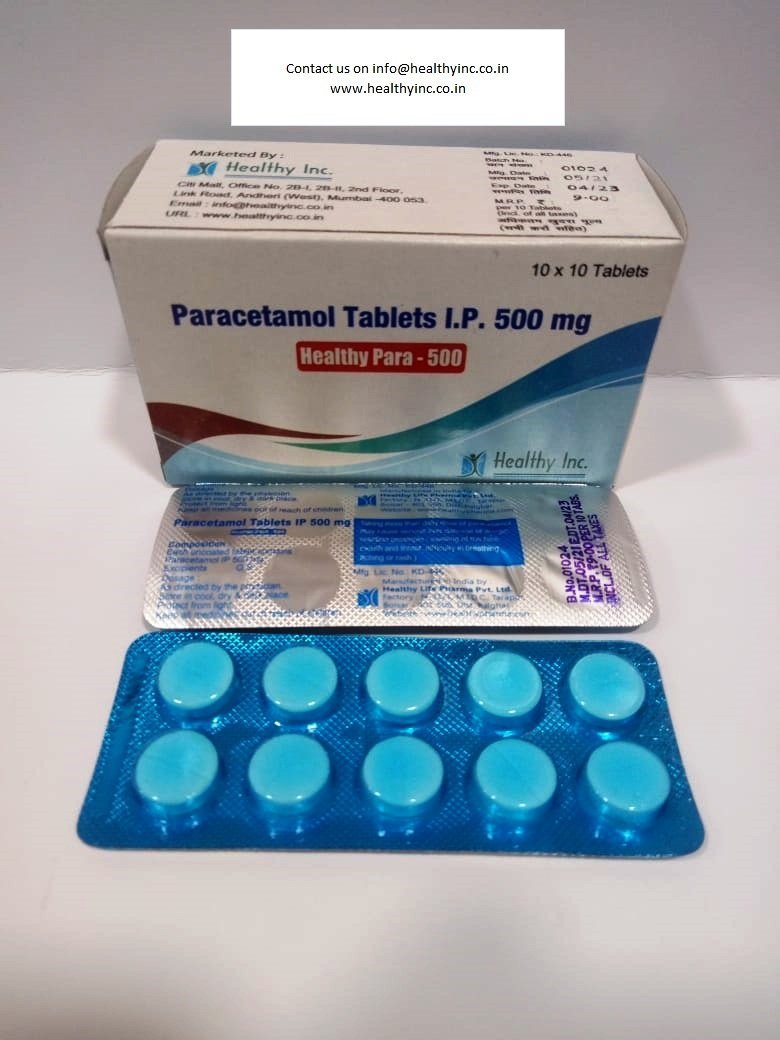
Ondansetron Orally Disintegrating Tablets (4 mg / 8 mg)
Healthy Inc is a specialized global supplier of high-performance antiemetic therapies. We provide premium Ondansetron Orally Disintegrating Tablets (ODT), manufactured in WHO–GMP certified specialized solid dosage units. Designed for rapid relief when swallowing is difficult, this product is a cornerstone export for oncology centers, pediatric wards, and emergency medical services across Southeast Asia, Africa, and Europe.
Product Overview
Ondansetron is a highly selective serotonin 5-HT3 receptor antagonist. The ODT (Mouth-Dissolving) variant is specifically engineered for patients suffering from severe nausea, where traditional tablet ingestion is hindered by emesis or dysphagia.
The “Rapid-Response” Antiemetic Specialist:
- Mechanism of Action: Ondansetron works by blocking 5-HT3 receptors located peripherally on vagal nerve terminals and centrally in the Chemoreceptor Trigger Zone (CTZ). By inhibiting serotonin binding at these sites, it effectively interrupts the signaling pathway that triggers the vomiting reflex.
- ODT Advantage: Our “Melt-in-Mouth” technology allows the tablet to disintegrate on the tongue within seconds without the need for water. This ensures immediate drug availability and prevents the patient from vomiting back the medication, a common failure point in oral antiemetic therapy.
- Clinical Gold Standard: It is the first-line choice for Chemotherapy-Induced Nausea and Vomiting (CINV), Radiotherapy-Induced Nausea, and Post-Operative Nausea and Vomiting (PONV).
Technical & Manufacturing Specifications
Formulated with advanced freeze-drying or low-pressure compression for instant dispersion.
| Technical Metric | Specification Standard |
|---|---|
| Active Ingredient | Ondansetron (as Base or HCl) BP / USP / IP |
| Dosage Form | Orally Disintegrating Tablets (ODT / MDT) |
| Disintegration Time | < 30 Seconds (Standardized) |
| HS Code | 3004.90.99 (Medicaments) / 2933.99.00 |
| Packaging | Alu-Alu Peel-Push Blister (Critical for fragile ODT structures) |
Manufacturing Authority
Marketed and Distributed by Healthy Inc from specialized ISO 9001:2015 units.
- Taste-Masking Excellence: Ondansetron is naturally bitter. We utilize micro-encapsulation and premium sweeteners to ensure a pleasant strawberry or mint profile, which is vital for pediatric compliance.
- Hygroscopic Protection: ODT formulations are highly sensitive to environmental moisture. Our Alu-Alu cold-form blisters provide an absolute barrier, ensuring the tablets remain structurally sound and chemically potent for up to 36 months.
- Dose Uniformity: Using high-precision direct compression, we ensure that every 4mg or 8mg tablet delivers an exact therapeutic dose, validated by rigorous HPLC assay testing.
Therapeutic Indications & Clinical Symptoms
- Cytotoxic Chemotherapy: Prevention of acute and delayed nausea and vomiting associated with highly or moderately emetogenic cancer treatments.
- Post-Operative Recovery: Prevention and treatment of nausea following general anesthesia and surgical procedures.
- Gastroenteritis: Used off-label in emergency settings to prevent dehydration in children by stopping persistent vomiting.
Dosage & Administration
- Standard Adult Dose: 8 mg ODT, usually taken 30 minutes before chemotherapy.
- Administration: Place the tablet on the tongue, allow it to dissolve (do not chew), and swallow with saliva. Water is not required.
- Safety Note: Use with caution in patients with congenital long QT syndrome, as 5-HT3 antagonists can affect cardiac conduction.
Global Export & Contract Manufacturing Services
Healthy Inc is a verified Pharmaceutical Exporter in Mumbai, catering to Oncology Hospital Chains and Ministry of Health Tenders. We offer Third Party Manufacturing for Ondansetron ODT with full CTD Dossier and COPP support. Our logistics network ensures secure transit to Vietnam, Nigeria, UK, and the Philippines, providing WHO-GMP quality at competitive B2B prices.
Commercial Inquiries
WhatsApp/Call: +91 7710003340
Email: info@healthyinc.co.in