Description
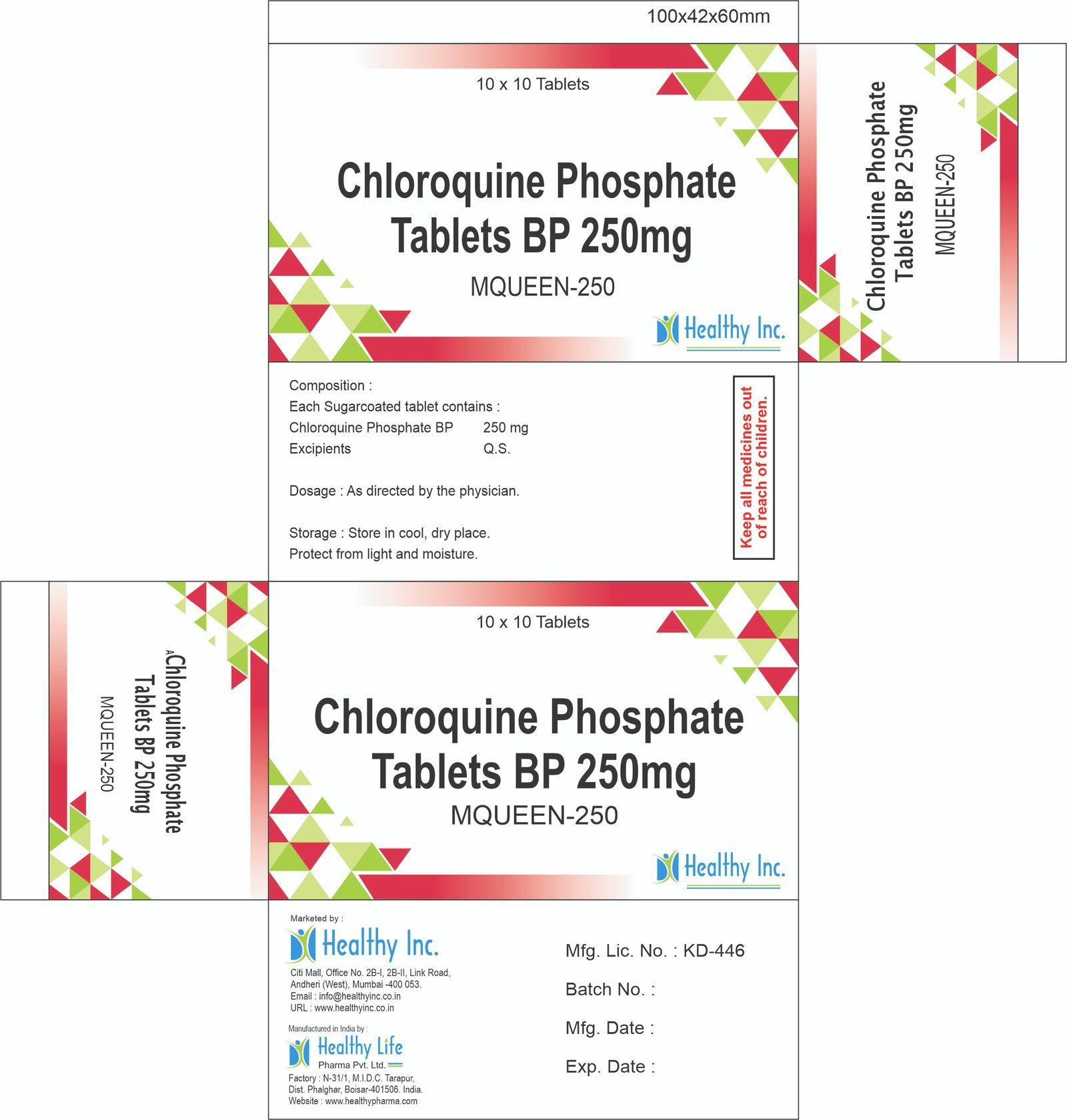
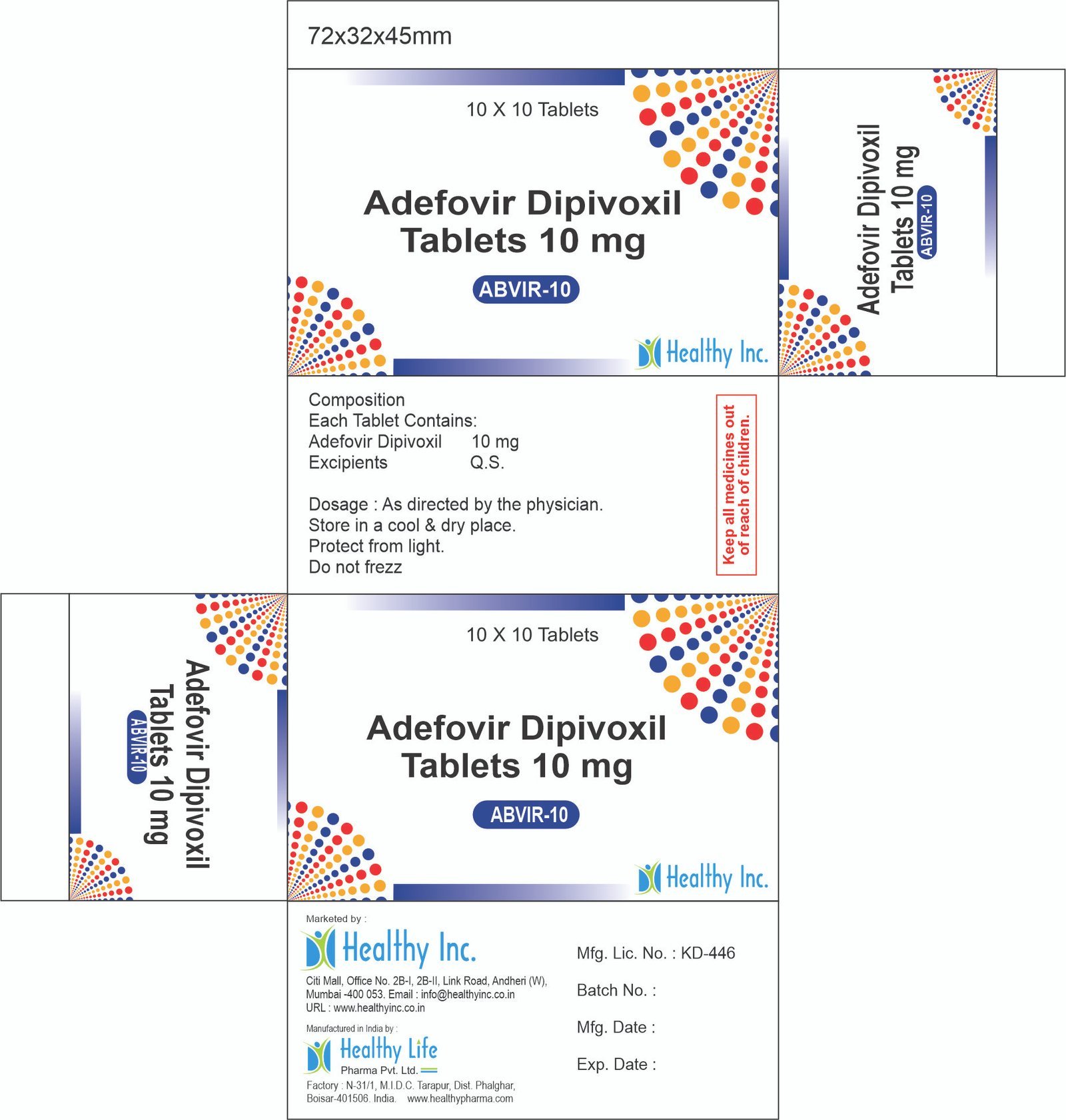
α – β Arteether Injection (Antimalarial)
Healthy Inc is a frontline defender in the global fight against Malaria. We provide high-quality α – β Arteether Injection, manufactured in our WHO–GMP certified facilities. This oil-based artemisinin derivative is a life-saving drug exported extensively to Africa, Southeast Asia, and LATAM for the treatment of severe, multi-drug resistant malaria.
Product Overview
α – β Arteether is an ethyl ether derivative of Artemisinin. It is a potent schizonticide that acts rapidly to clear parasitemia from the blood.
It is the “Drug of Choice” for:
- Severe Malaria: Caused by Plasmodium falciparum strains resistant to Chloroquine and other antimalarials.
- Cerebral Malaria: A severe neurological complication of malaria infection that requires immediate intervention.
- Rapid Clearance: Known for its ability to clear fever and parasites faster than many older antimalarials.
Product Composition & Strength
We supply this product in an Oily Base formulation for deep Intramuscular (IM) use.
| Active Ingredient | Strength | Presentation |
|---|---|---|
| α – β Arteether | 150 mg / 2 ml | 2ml Ampoule (75 mg/ml) |
*Formulated in refined Arachis Oil / Sesame Oil base.
Technical & Logistics Specifications
Critical data for Pharmaceutical Importers and Government Tenders.
| HS Code | 3004.90.99 (Antimalarial Medicaments) |
| Dosage Form | Oily Injection (Parenteral) |
| Route of Administration | Deep Intramuscular (IM) ONLY |
| Packaging | 3 x 2ml Ampoules (Tray/Blister Pack) |
| Storage | Store below 30°C. Protect from light. |
| Certificates | WHO-GMP, COPP, FSC, CTD Dossier |
Manufacturing Authority
Manufactured at our WHO-GMP & ISO 9001:2015 certified unit.
- Technology: Dedicated filling lines for oily injectables to ensure precise volume and sterility.
- Quality: Strict testing for related substances and impurities to ensure low toxicity.
- Capacity: Large-scale production capable of meeting seasonal malaria peak demands.
Therapeutic Indications
Indicated for the curative treatment of:
- Severe Plasmodium falciparum malaria.
- Cerebral Malaria (Coma associated with malaria).
- Malaria in patients where oral administration is not feasible (vomiting/unconscious).
Dosage & Administration
Strict Medical Supervision Required:
- Adults: 150 mg (1 ampoule) via Deep Intramuscular injection daily for 3 consecutive days. (Total dose: 450 mg).
- Children: 3 mg/kg body weight daily for 3 days.
- Injection Site: Gluteal muscle (Buttocks).
Safety Warnings:
- IM ONLY: This is an oily injection. NEVER administer Intravenously (IV) as it can cause embolism.
- Pregnancy: Use in pregnancy only if the potential benefit justifies the potential risk to the fetus (WHO Guidelines).
Commercial Inquiries
For government malaria tenders, NGO supplies, or distributor pricing, please contact our export team.
WhatsApp/Call: +91 7710003340
Email: info@healthyinc.co.in