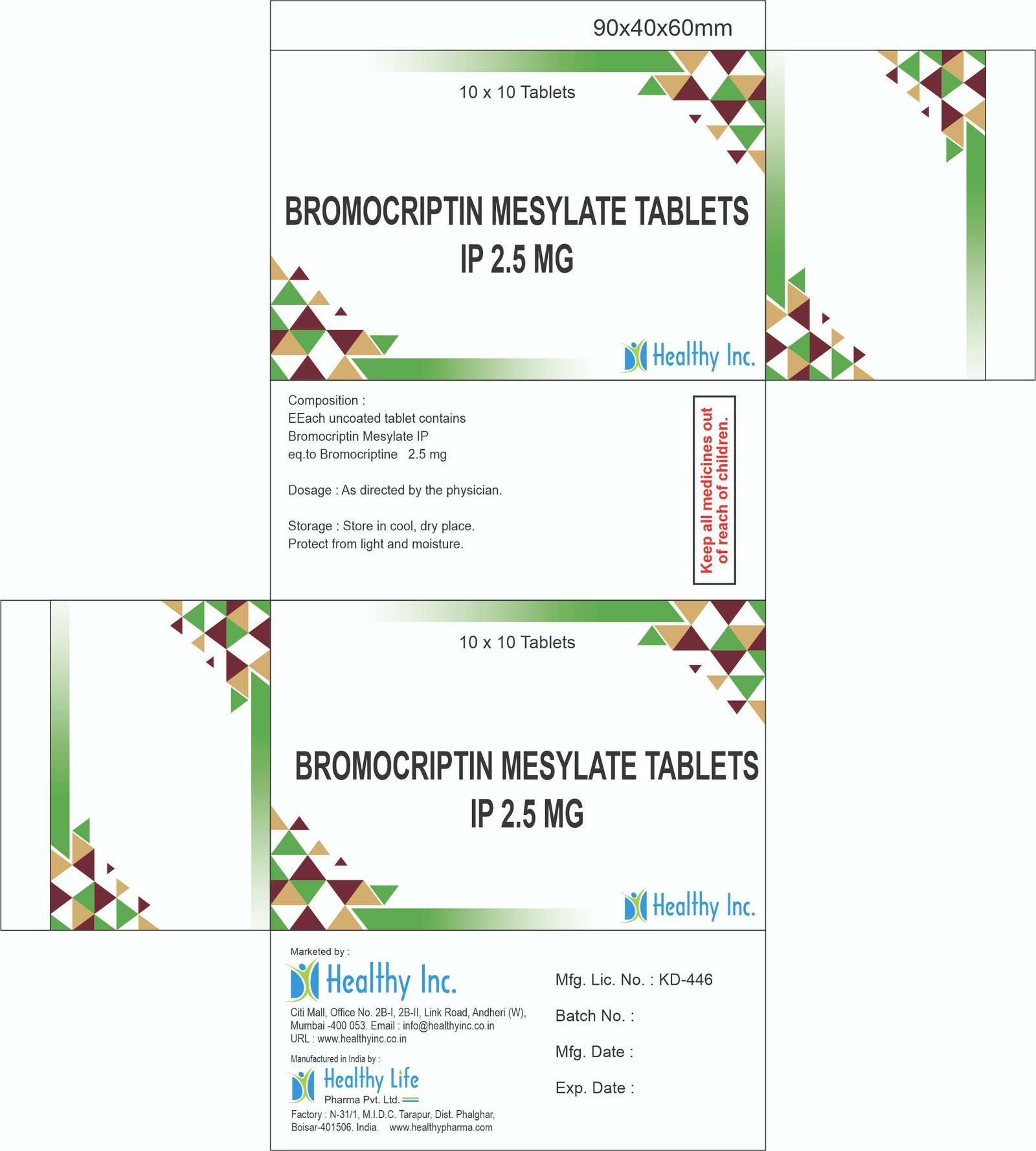
Description
Amodiaquine Hydrochloride Tablets
Healthy Inc is a specialized global supplier and exporter of essential antimalarial pharmacotherapies. We provide high-purity Amodiaquine Hydrochloride Tablets, sourced from WHO–GMP certified solid dosage facilities. This “4-Aminoquinoline” agent is a top export to National Malaria Control Programs (NMCP), NGO humanitarian missions, and Ministry of Health tenders in Africa (West & Central) and Southeast Asia, serving as the critical partner drug in Artemisinin-Based Combination Therapy (ACT) for the treatment of uncomplicated malaria.
[Image of Amodiaquine mechanism inhibiting heme polymerization in Plasmodium food vacuole]
Product Overview
This formulation contains Amodiaquine Hydrochloride, a potent antimalarial chemically related to Chloroquine but effective against many Chloroquine-resistant strains.
The “Resistance Rescue” Specialist:
- Mechanism: Like other quinolines, Amodiaquine accumulates in the “food vacuole” of the malaria parasite (Plasmodium falciparum). It interferes with the parasite’s ability to detoxify hemoglobin by-products. By inhibiting the polymerization of toxic heme into non-toxic hemozoin, it causes the parasite to be poisoned by its own metabolic waste.
- ACT Partner: While rarely used alone due to resistance concerns, Amodiaquine is the preferred long-acting partner for Artesunate. The Artesunate rapidly reduces the parasite load, while Amodiaquine remains in the blood longer to kill residual parasites and prevent recrudescence.
- Efficacy: It remains highly effective in regions where Chloroquine has failed, particularly in West and Central Africa.
Product Composition & Strength
We supply this product as Uncoated Tablets (Yellow) or Film Coated Tablets. The strength is typically expressed in terms of the Amodiaquine Base.
| Active Ingredient | Strength (Base Equivalent) | Salt Content (Approx) | Role |
|---|---|---|---|
| Amodiaquine HCl USP/BP | 67.5 mg | ~ 90 mg | Pediatric (ACT Component) |
| Amodiaquine HCl USP/BP | 153 mg / 150 mg | ~ 200 mg | Adolescent / Co-blistered |
| Amodiaquine HCl USP/BP | 200 mg | ~ 260 mg | Standard Adult Dose |
| Excipients | Q.S. | Microcrystalline Cellulose / Magnesium Stearate | Direct Compression |
*Pack Sizes: Blister packs of 10s (Box of 10×10), 3s (Course Therapy), or Co-blistered with Artesunate.
Technical & Logistics Specifications
Critical data for Pharmaceutical Importers and Distributors.
| HS Code | 3004.90.99 (Other Medicaments) |
| Dosage Form | Tablet (Uncoated – Yellow) |
| Packaging | PVC-Alu Blister or Alu-Alu Blister (Light Sensitive) |
| Storage | Store below 30°C. Protect from Light. |
| Certificates | WHO-GMP, COPP, Free Sale Certificate |
Manufacturing Authority
Marketed and Distributed by Healthy Inc from WHO-GMP & ISO 9001:2015 certified units.
- Photostability: Amodiaquine is sensitive to light. Exposure causes the yellow tablet to darken to brown, indicating degradation. We strictly use amber-colored PVC or opaque Alu-Alu blisters to guarantee shelf-life stability in field conditions.
- Friability & Hardness: For bulk tenders supplied in jars or large packs, we engineer tablets with high hardness to withstand rough transport conditions on unpaved roads in remote distribution areas.
Therapeutic Indications (Human Use)
Indicated for the treatment of malaria:
- Uncomplicated Malaria: Treatment of acute uncomplicated P. falciparum malaria.
- Combination Therapy: Must be used in combination with Artesunate to prevent the development of resistance (Artesunate + Amodiaquine = ASAQ).
- Note: Not recommended for chemoprophylaxis (prevention) due to liver toxicity risks with long-term use.
Dosage & Administration
Recommended Dosage (Strictly as per Physician/WHO Guidelines):
- Route: Oral.
- Standard Adult Dosage: 10 mg/kg body weight once daily for 3 days.
- Total Course Dose: 30 mg/kg over 3 days.
- Example (Adult 60kg): Two 300mg tablets (or three 200mg tablets) taken once daily for 3 days.
- Administration: Can be crushed and mixed with water for children.
Safety Warnings:
- Hepatotoxicity: Rare but serious liver toxicity can occur. Monitor for jaundice (yellow eyes/skin).
- Agranulocytosis: Can lower white blood cell count (neutropenia). Patients should report severe sore throat or fever immediately.
- Neurological: Can cause involuntary movements (extrapyramidal symptoms) in rare cases.
- Overdose: Acute overdose is dangerous and can cause cardiac arrest. Immediate medical attention is required.
Global Export & Contract Manufacturing Services
Healthy Inc stands as a premier Pharmaceutical Exporter in India, dedicated to serving the needs of international Pharma Traders, Wholesalers, and Bulk Drug Distributors. As a verified Medicine Supplier in Mumbai, we offer flexible Third Party Manufacturing (Contract Manufacturing) services for OSD (Oral Solid Dosage) forms, allowing brands to launch high-quality generic medicines under their own label. Whether you are looking for a reliable Hospital Tender Supplier for government procurement in Africa or a B2B Pharma Marketplace partner for Latin America, our logistics network ensures timely delivery. We actively support Pharmaceutical Drop Shipping models and bulk indenting, ensuring that every Generic Medicine Wholesaler receives WHO-GMP certified products at competitive rates.
Commercial Inquiries
For hospital tenders, bulk export, or distributor pricing, please contact our export team.
WhatsApp/Call: +91 7710003340
Email: info@healthyinc.co.in