Description
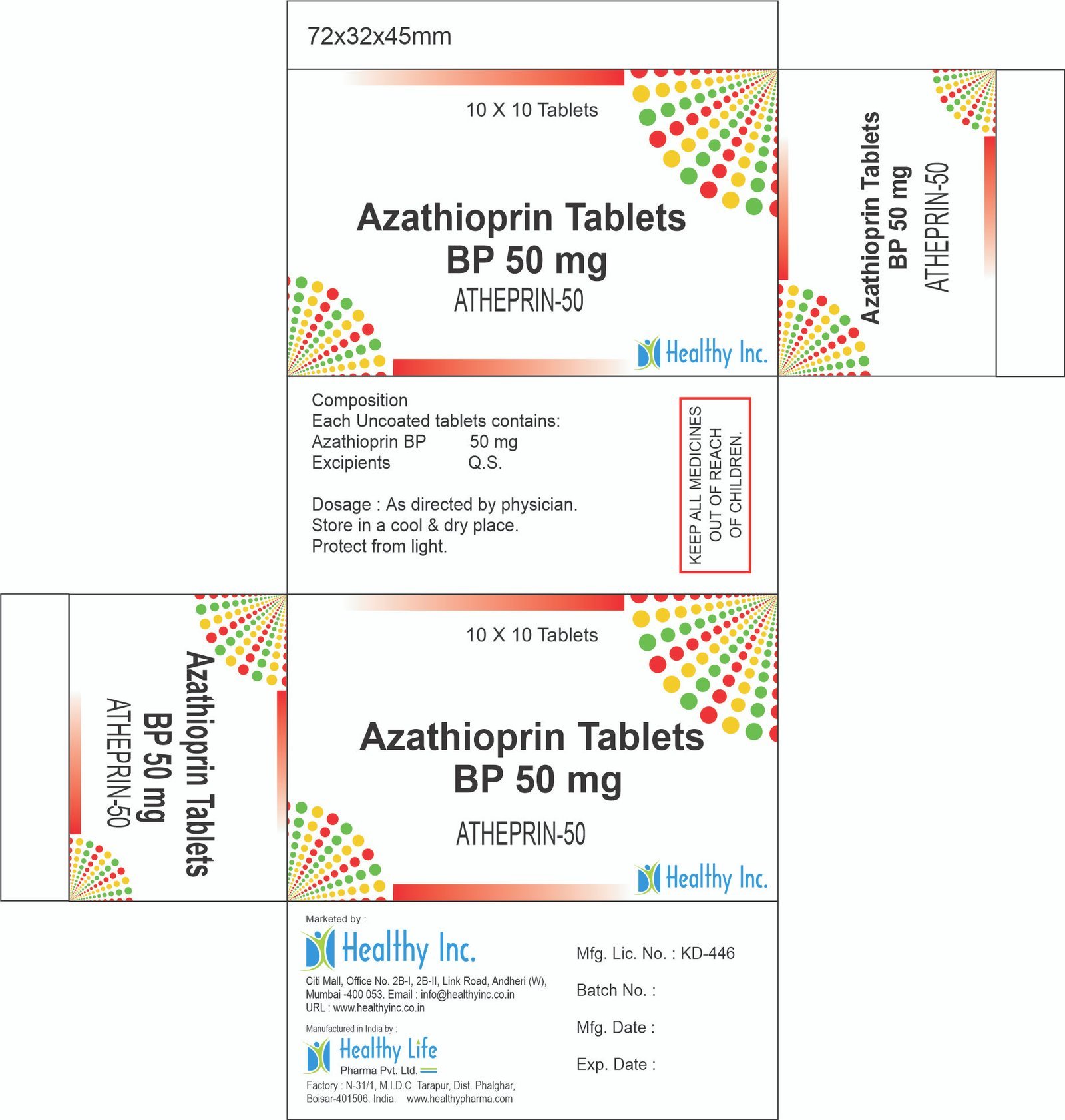
Azathioprine Tablets
Healthy Inc is a specialized global supplier and exporter of critical immunosuppressive agents. We provide high-purity Azathioprine Tablets, sourced from WHO–GMP certified solid dosage facilities. This “Steroid-Sparing” agent is a top export to organ transplant centers, rheumatology departments, and gastroenterology clinics in Africa, LATAM, and Southeast Asia, serving as a foundational therapy for preventing organ rejection and managing severe autoimmune disorders.
Product Overview
This formulation contains Azathioprine, a purine analogue and antimetabolite immunosuppressant.
The “Immune Modulator” Specialist:
- Mechanism: Azathioprine is a prodrug that is converted in the body to **6-mercaptopurine (6-MP)**. It works by inhibiting DNA and RNA synthesis specifically in T-lymphocytes and B-lymphocytes (white blood cells), thereby suppressing the overactive immune response.
- Steroid-Sparing Effect: When used in combination with corticosteroids (like Prednisolone), it allows doctors to significantly lower the steroid dose, reducing the risk of long-term steroid side effects (osteoporosis, weight gain).
- Transplant Survival: Essential for preventing the body from rejecting transplanted kidneys, livers, or hearts.
- Gut Health: A key maintenance drug for inducing and maintaining remission in inflammatory bowel diseases (Crohn’s & Colitis).
Product Composition & Strength
We supply this product as Film Coated Tablets (Yellow/Pale Yellow) in blister packs or bulk containers.
| Active Ingredient | Strength (Standard) | Role |
|---|---|---|
| Azathioprine USP/BP | 50 mg | Standard Adult Dose |
| Azathioprine USP/BP | 25 mg | Low Dose / Renal Adjustment |
| Excipients | Q.S. | Binder / Coating Agent |
*Pack Sizes: Blister packs of 10s (Box of 5×10 or 10×10) or Bulk HDPE Jars of 100s.
Technical & Logistics Specifications
Critical data for Pharmaceutical Importers and Distributors.
| HS Code | 3004.90.99 (Other Medicaments) |
| Dosage Form | Film Coated Tablet (Oral) |
| Packaging | PVC-Alu Blister or Alu-Alu Blister (Light Sensitive) |
| Storage | Store below 25°C. Protect from Light. |
| Certificates | WHO-GMP, COPP, Free Sale Certificate |
Manufacturing Authority
Marketed and Distributed by Healthy Inc from WHO-GMP & ISO 9001:2015 certified units.
- Cytotoxic Handling: Azathioprine is classified as a hazardous drug. It is manufactured in dedicated Oncology/Cytotoxic blocks with negative pressure containment to prevent cross-contamination with general medicines.
- Light Stability: The API is sensitive to light. We use opaque blister packaging and amber coating to ensure stability.
Therapeutic Indications (Human Use)
Indicated for immunosuppression in:
- Organ Transplantation: Prophylaxis of rejection in renal homotransplantation (Kidney Transplant).
- Rheumatoid Arthritis: Management of severe, active rheumatoid arthritis unresponsive to conventional therapy.
- Systemic Lupus Erythematosus (SLE): Autoimmune control.
- Inflammatory Bowel Disease: Crohn’s Disease and Ulcerative Colitis (maintenance of remission).
Dosage & Administration
Recommended Dosage (Strictly as per Transplant Surgeon/Rheumatologist):
- Route: Oral.
- Transplantation: Initial dose 3 to 5 mg/kg daily, tapering to maintenance of 1 to 3 mg/kg daily.
- Rheumatoid Arthritis / IBD: Initial dose 1 mg/kg (approx. 50–100 mg) daily, administered in one or two doses.
- Administration: Take with food to reduce nausea. Tablets should not be divided or crushed by pregnant caregivers (hazardous dust).
Safety Warnings:
- Bone Marrow Suppression: Can cause severe leukopenia (low white blood cells) and thrombocytopenia. Weekly blood counts are required during initiation.
- Drug Interaction (Critical): Allopurinol (Gout medicine) blocks the breakdown of Azathioprine. If taken together, the Azathioprine dose must be reduced to 25% of the original dose to prevent fatal toxicity.
- TPMT Deficiency: Patients with Thiopurine S-methyltransferase (TPMT) deficiency are at high risk of toxicity.
Global Export & Contract Manufacturing Services
Healthy Inc stands as a premier Pharmaceutical Exporter in India, dedicated to serving the needs of international Pharma Traders, Wholesalers, and Bulk Drug Distributors. As a verified Medicine Supplier in Mumbai, we offer flexible Third Party Manufacturing (Contract Manufacturing) services for OSD (Oral Solid Dosage) forms, allowing brands to launch high-quality generic medicines under their own label. Whether you are looking for a reliable Hospital Tender Supplier for government procurement in Africa or a B2B Pharma Marketplace partner for Latin America, our logistics network ensures timely delivery. We actively support Pharmaceutical Drop Shipping models and bulk indenting, ensuring that every Generic Medicine Wholesaler receives WHO-GMP certified products at competitive rates.
Commercial Inquiries
For hospital tenders, bulk export, or distributor pricing, please contact our export team.
WhatsApp/Call: +91 7710003340
Email: info@healthyinc.co.in