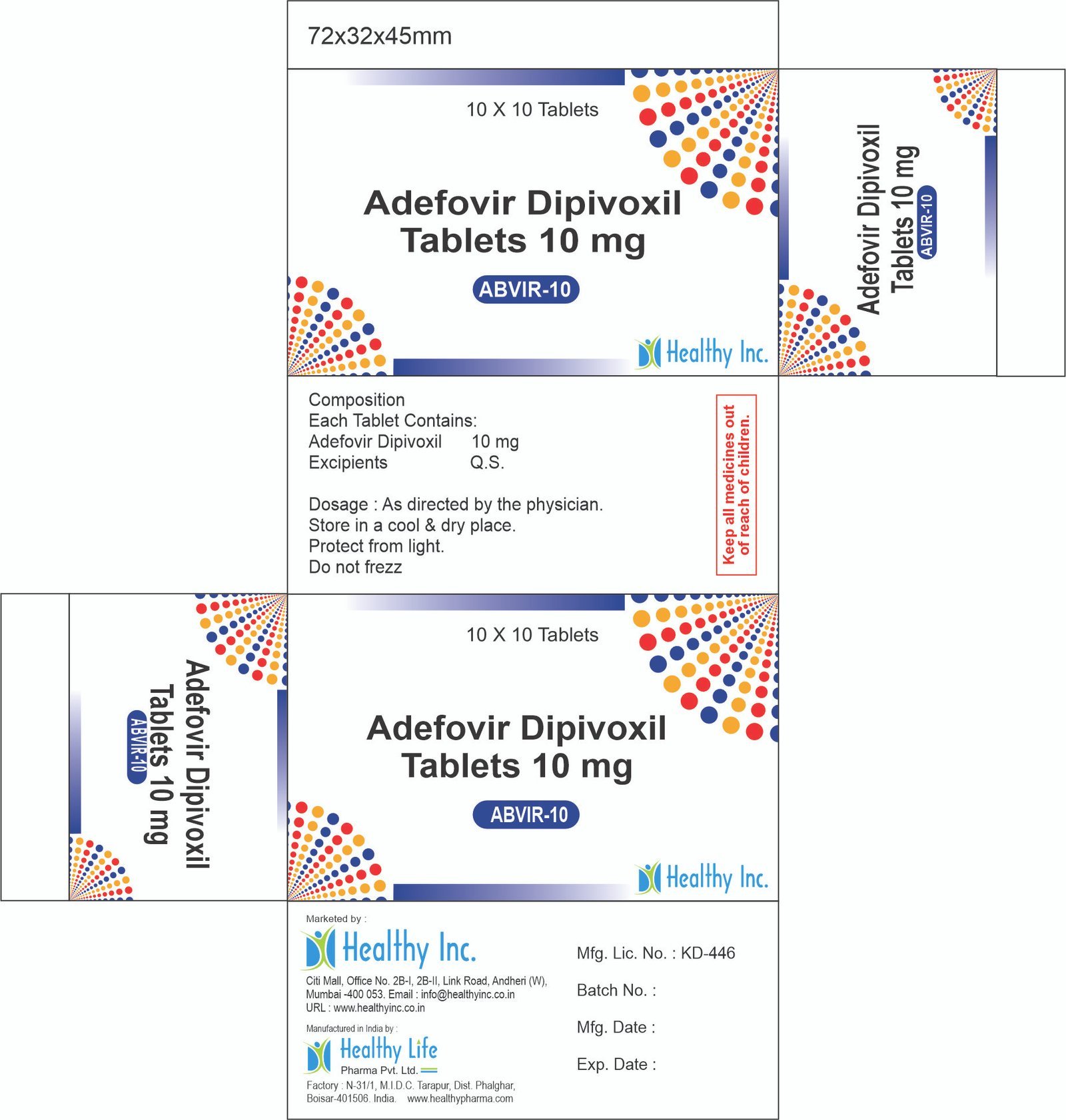
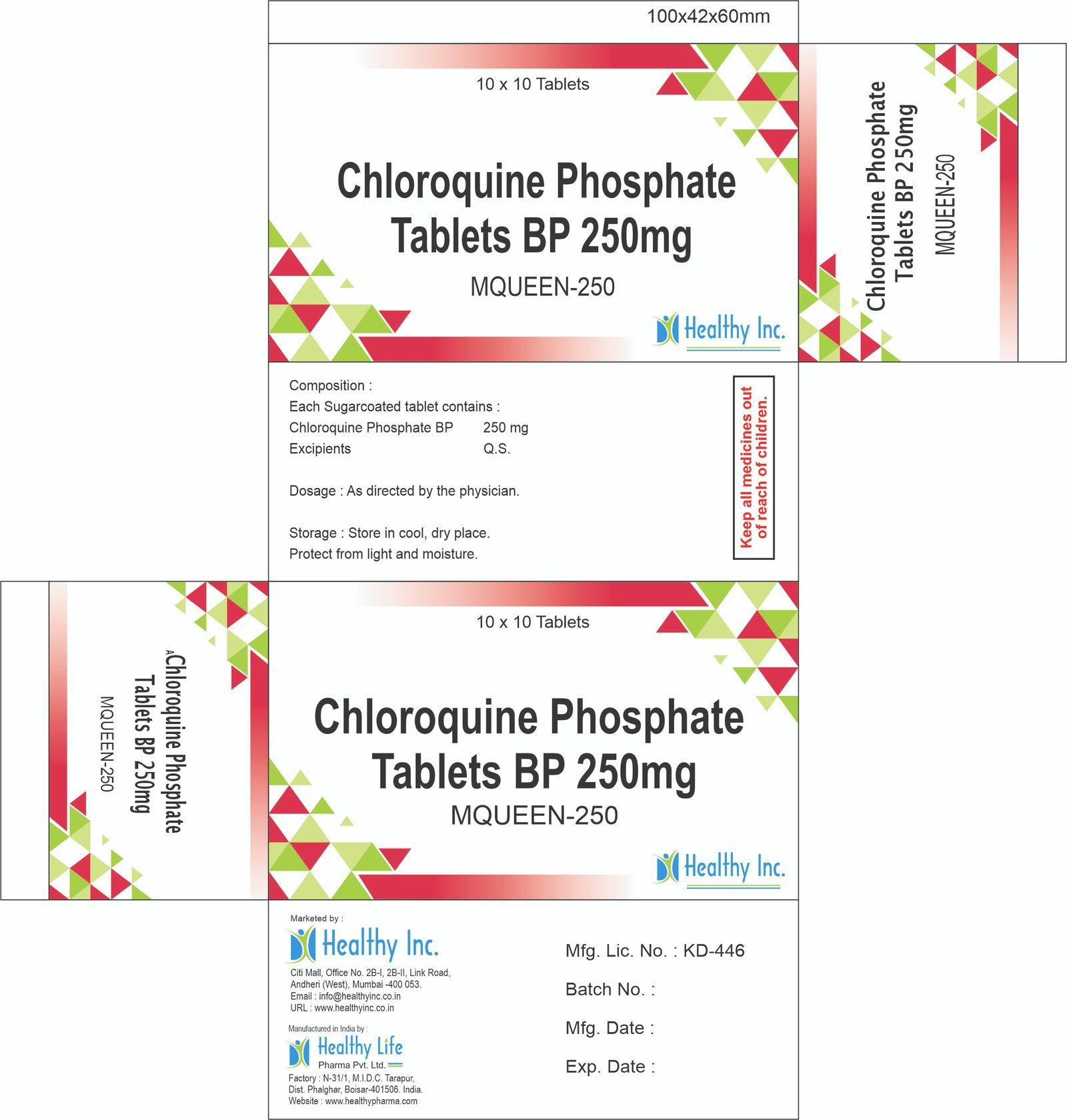
Description
Azithromycin Dispersible Tablets
Healthy Inc is a specialized global supplier and exporter of advanced macrolide antibiotics. We provide high-purity Azithromycin Dispersible Tablets (DT), sourced from WHO–GMP certified solid dosage facilities. This “Short-Course” antibiotic is a top export to pediatric clinics, ENT departments, and government health tenders in Africa, LATAM, and Southeast Asia, serving as the preferred choice for treating respiratory tract infections and enteric fever in children due to its high compliance profile.
Product Overview
This formulation contains Azithromycin (as Dihydrate), a broad-spectrum azalide antibiotic (subclass of macrolides).
The “Tissue-Directed” Specialist:
- Mechanism: It binds irreversibly to the **50S ribosomal subunit** of susceptible bacteria, blocking protein synthesis and inhibiting cell growth (bacteriostatic/bactericidal).
- Tissue Concentration: Unlike older antibiotics that stay in the blood, Azithromycin rapidly leaves the bloodstream and concentrates in tissues and white blood cells (phagocytes), which carry the drug directly to the site of infection.
- Post-Antibiotic Effect: Because it stays in the tissues for days, a short **3-day course** is effectively equivalent to a 10-day course of Amoxicillin.
- Pediatric Compliance: The dispersible form is specifically designed for children who cannot swallow pills, allowing the tablet to dissolve in a spoon of water.
Product Composition & Strength
We supply this product as Dispersible Tablets (DT) with advanced taste-masking technology.
| Active Ingredient | Strength | Flavor Profile | Role |
|---|---|---|---|
| Azithromycin IP/BP/USP | 100 mg | Orange / Peppermint | Pediatric Low Dose |
| Azithromycin IP/BP/USP | 250 mg | Orange / Peppermint | Pediatric Standard / Adult |
| Excipients | Q.S. | Sweeteners | Disintegrant / Flavor |
*Pack Sizes: Blister packs of 3s, 6s (Common courses) or 10s.
Technical & Logistics Specifications
Critical data for Pharmaceutical Importers and Distributors.
| HS Code | 3004.20.19 (Medicaments containing other antibiotics) |
| Dosage Form | Dispersible Tablet (Uncoated) |
| Packaging | PVC-Alu Blister or Alu-Alu Blister |
| Storage | Store below 30°C. Protect from moisture. |
| Certificates | WHO-GMP, COPP, Free Sale Certificate |
Manufacturing Authority
Marketed and Distributed by Healthy Inc from WHO-GMP & ISO 9001:2015 certified units.
- Taste Masking: Azithromycin is extremely bitter. We use ion-exchange resins or micro-encapsulation techniques to mask the bitterness, ensuring children do not spit out the dose.
- Dispersion Time: Formulated to disintegrate in less than 60 seconds in a small amount of water (approx. 10ml).
Therapeutic Indications (Human Use)
Indicated for mild to moderate infections:
- Respiratory Tract: Acute bacterial sinusitis, Pharyngitis/Tonsillitis (Strep throat), and Otitis Media (Ear infection).
- Enteric Fever: Uncomplicated Typhoid fever (caused by Salmonella typhi).
- Skin Infections: Uncomplicated skin and skin structure infections.
Dosage & Administration
Recommended Dosage (Strictly as per Pediatrician/Physician):
- Route: Oral (Dissolved in water).
- General Pediatric Dose: 10 mg/kg body weight once daily for 3 days.
- Typhoid Fever: 20 mg/kg once daily for 5–7 days.
- Administration: Dissolve the tablet in a teaspoon of boiled and cooled water. Take 1 hour before or 2 hours after meals (food decreases absorption of some formulations).
Safety Warnings:
- QT Prolongation: Can cause abnormal heart rhythm (arrhythmia). Caution in patients with existing heart conditions.
- Liver Function: Excreted via the liver; discontinue if signs of jaundice or hepatitis appear.
- Diarrhea: Common side effect; usually mild and resolves after treatment stops.
Global Export & Contract Manufacturing Services
Healthy Inc stands as a premier Pharmaceutical Exporter in India, dedicated to serving the needs of international Pharma Traders, Wholesalers, and Bulk Drug Distributors. As a verified Medicine Supplier in Mumbai, we offer flexible Third Party Manufacturing (Contract Manufacturing) services for OSD (Oral Solid Dosage) forms, allowing brands to launch high-quality generic medicines under their own label. Whether you are looking for a reliable Hospital Tender Supplier for government procurement in Africa or a B2B Pharma Marketplace partner for Latin America, our logistics network ensures timely delivery. We actively support Pharmaceutical Drop Shipping models and bulk indenting, ensuring that every Generic Medicine Wholesaler receives WHO-GMP certified products at competitive rates.
Commercial Inquiries
For hospital tenders, bulk export, or distributor pricing, please contact our export team.
WhatsApp/Call: +91 7710003340
Email: info@healthyinc.co.in