Description
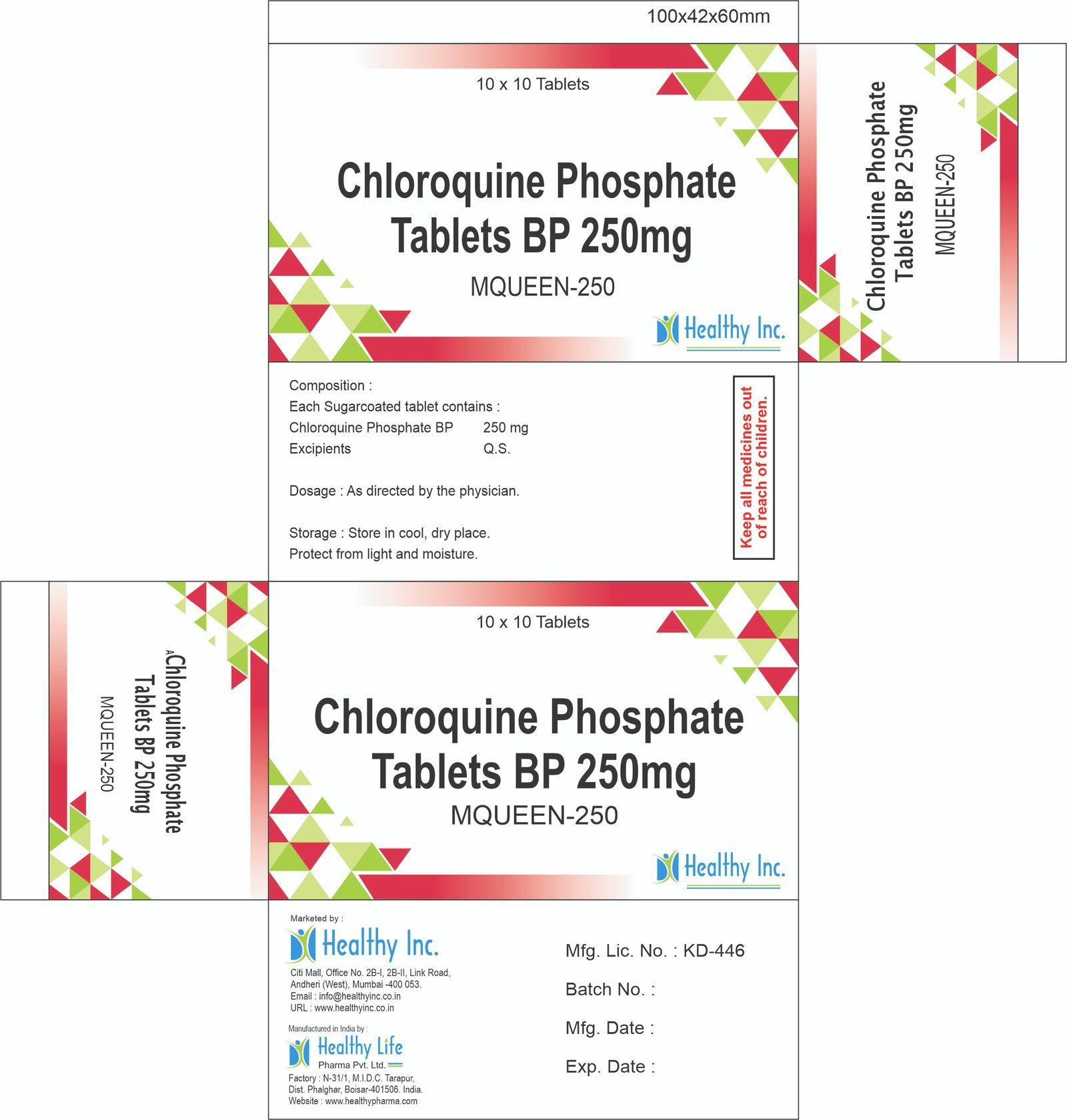
Chloroquine Phosphate Tablets
Healthy Inc is a specialized global supplier and exporter of essential antimalarial and autoimmune therapeutics. We provide high-purity Chloroquine Phosphate Tablets, sourced from WHO–GMP certified solid dosage facilities. This “Time-Tested” 4-Aminoquinoline is a top export to National Malaria Control Programs, rheumatology clinics, and government health tenders in Africa, LATAM, and Southeast Asia, recognized for its dual efficacy in treating non-resistant Malaria and chronic autoimmune conditions like Lupus and Rheumatoid Arthritis.
Product Overview
This formulation contains Chloroquine Phosphate, a synthetic antimalarial and Disease-Modifying Anti-Rheumatic Drug (DMARD).
The “Heme Blocker” Specialist:
- Antimalarial Mechanism: Malaria parasites digest hemoglobin inside red blood cells, releasing toxic “Heme.” They usually convert this toxin into harmless hemozoin. Chloroquine blocks this conversion (polymerization), causing toxic heme to build up and kill the parasite. It is highly effective against the blood stages of *Plasmodium vivax*, *P. ovale*, *P. malariae*, and sensitive strains of *P. falciparum*.
- Anti-Inflammatory Mechanism: In autoimmune diseases (Lupus/RA), Chloroquine accumulates in lysosomes, increasing pH and interfering with antigen processing. This reduces the immune system’s attack on the body’s own tissues.
- Tissue Amebiasis: It concentrates in the liver, making it effective for treating extra-intestinal amebiasis (Amebic Hepatitis) when other drugs fail.
Product Composition & Strength
We supply this product as Film Coated Tablets. The coating is critical to mask the intensely bitter taste of the drug. Note: Dosing is often calculated based on the “Base” content.
| Active Ingredient (Salt) | Equivalent Base | Primary Role |
|---|---|---|
| Chloroquine Phosphate USP/BP 250 mg | ~ 155 mg Base | Prophylaxis / Maintenance |
| Chloroquine Phosphate USP/BP 500 mg | ~ 310 mg Base | Acute Malaria Treatment |
| Excipients | Q.S. | Starch / Magnesium Stearate |
*Pack Sizes: Blister packs of 10s (Box of 10×10), 1000s Bulk HDPE Jars (Hospital/Institutional Packs).
Technical & Logistics Specifications
Critical data for Pharmaceutical Importers and Distributors.
| HS Code | 3004.60.00 (Medicaments containing antimalarial principles) |
| CAS Number | 50-63-5 |
| Dosage Form | Film Coated Tablet (Oral) |
| Packaging | PVC-Alu Blister or Alu-Alu Blister |
| Storage | Store below 30°C. Protect from light. |
| Certificates | WHO-GMP, COPP, Free Sale Certificate |
Manufacturing Authority
Marketed and Distributed by Healthy Inc from WHO-GMP & ISO 9001:2015 certified units.
- Taste Masking (Critical): Chloroquine is notoriously bitter. If a tablet is uncoated or the coating is poor, it can induce immediate vomiting (emesis), rendering the dose ineffective. We use a robust Opadry Film Coating that ensures the tablet passes the taste barrier intact.
- Dissolution: Formulated for immediate release to rapidly achieve therapeutic blood levels necessary to reduce parasite load during the febrile (fever) stage.
Therapeutic Indications (Human Use)
Indicated for the treatment and suppression of parasitic and autoimmune diseases:
- Malaria Treatment: Acute attacks of malaria due to *P. vivax*, *P. malariae*, *P. ovale*, and sensitive *P. falciparum*.
- Malaria Prophylaxis: Suppressive treatment to prevent malaria in travelers to endemic areas.
- Extra-intestinal Amebiasis: Treatment of amebic liver abscess.
- Autoimmune Disorders: Rheumatoid Arthritis (RA) and Systemic/Discoid Lupus Erythematosus (SLE/DLE) (as a second-line agent to Hydroxychloroquine).
Dosage & Administration
Recommended Dosage (Strictly as per Physician/WHO Guidelines):
- Route: Oral.
- Malaria Treatment (Standard Adult):
- Initial: 1000 mg (salt) immediately.
- 6 Hours later: 500 mg (salt).
- Day 2: 500 mg (salt).
- Day 3: 500 mg (salt).
- Total Dose: 2500 mg salt (~1.5 g base) over 3 days.
- Prophylaxis: 500 mg (salt) once weekly, on the same day each week. Start 1-2 weeks before travel and continue for 4 weeks after leaving.
- Lupus/RA: 250 mg to 500 mg daily (Maintenance dose should minimize ocular risk).
Safety Warnings:
- Ocular Toxicity (Retinopathy): Long-term use can cause irreversible retinal damage. Patients on chronic therapy (Lupus/RA) must have baseline and regular eye exams.
- Cardiac Effects: Can cause QT interval prolongation and arrhythmias. Use caution in patients with heart disease or electrolyte imbalances.
- G6PD Deficiency: May cause hemolysis (breaking of red blood cells) in G6PD-deficient patients.
- Psoriasis: Can exacerbate psoriasis flare-ups.
Global Export & Contract Manufacturing Services
Healthy Inc stands as a premier Pharmaceutical Exporter in India, dedicated to serving the needs of international Pharma Traders, Wholesalers, and Bulk Drug Distributors. As a verified Medicine Supplier in Mumbai, we offer flexible Third Party Manufacturing (Contract Manufacturing) services for OSD (Oral Solid Dosage) forms, allowing brands to launch high-quality generic medicines under their own label. Whether you are looking for a reliable Hospital Tender Supplier for government procurement in Africa or a B2B Pharma Marketplace partner for Latin America, our logistics network ensures timely delivery. We actively support Pharmaceutical Drop Shipping models and bulk indenting, ensuring that every Generic Medicine Wholesaler receives WHO-GMP certified products at competitive rates.
Commercial Inquiries
For hospital tenders, bulk export, or distributor pricing, please contact our export team.
WhatsApp/Call: +91 7710003340
Email: info@healthyinc.co.in