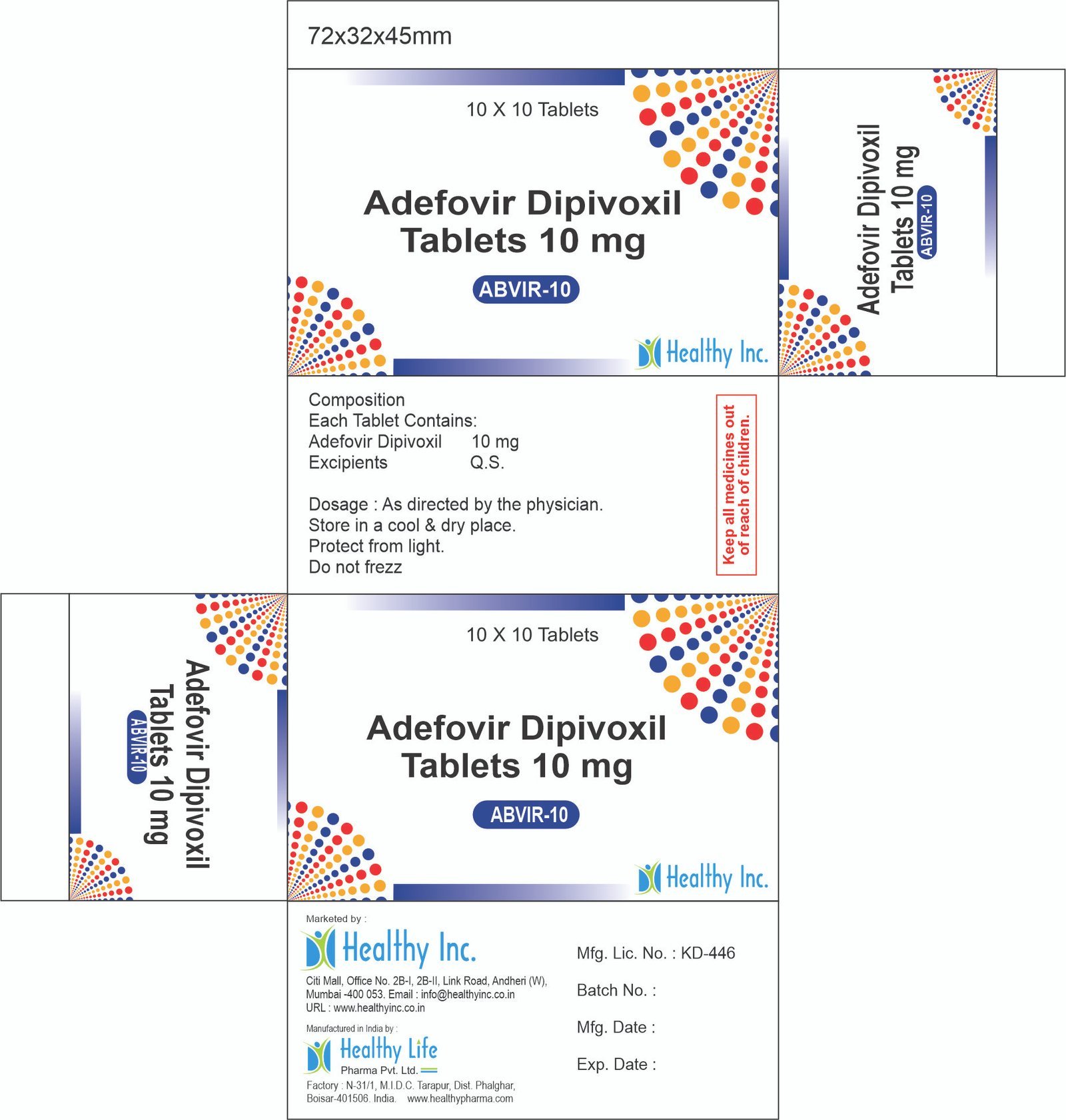
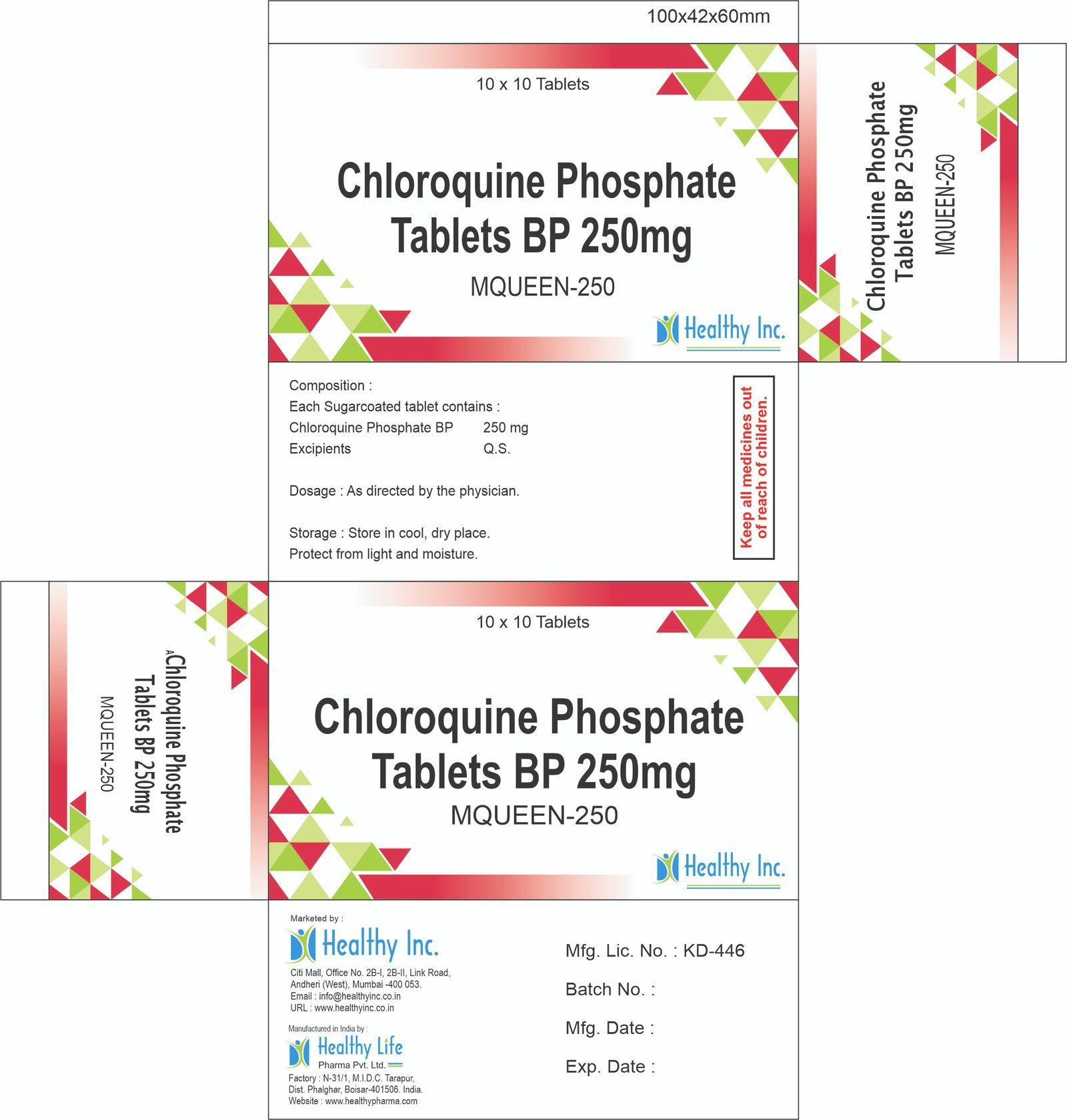
Description
Dapsone Tablets
Healthy Inc is a specialized global supplier and exporter of essential sulfone antibiotics. We provide high-purity Dapsone Tablets, sourced from WHO–GMP certified solid dosage facilities. This “Leprostatic” agent is a top export to National Leprosy Eradication Programs (NLEP), dermatology clinics, and HIV care centers in Africa, LATAM, and Southeast Asia, serving as the cornerstone of Multi-Drug Therapy (MDT) for Hansen’s Disease and the primary treatment for Dermatitis Herpetiformis.
Product Overview
This formulation contains Dapsone (Diaminodiphenyl Sulfone), a synthetic sulfone antimicrobial with powerful anti-inflammatory properties.
The “Dual-Role” Specialist:
- Mechanism (Antibacterial): Similar to sulfonamides, Dapsone inhibits the bacterial enzyme dihydropteroate synthase. This blocks the synthesis of dihydrofolic acid, starving the bacteria of the folate required for DNA synthesis. It is bacteriostatic against Mycobacterium leprae.
- Mechanism (Anti-Inflammatory): In skin conditions like Dermatitis Herpetiformis, Dapsone inhibits the migration of neutrophils (white blood cells) to the site of inflammation and prevents them from releasing damaging enzymes. This provides rapid relief from severe itching and blistering.
- Pneumocystis Prophylaxis: It is also used as a second-line prophylactic agent against Pneumocystis jirovecii pneumonia (PCP) in HIV/AIDS patients who cannot tolerate Trimethoprim-Sulfamethoxazole.
Product Composition & Strength
We supply this product as Uncoated Tablets (White). The range of strengths allows for precise dosing in both leprosy programs and dermatological maintenance.
| Active Ingredient | Strength (Standard) | Primary Role |
|---|---|---|
| Dapsone USP/BP | 25 mg | Pediatric / Initiation |
| Dapsone USP/BP | 50 mg | Intermediate Dose |
| Dapsone USP/BP | 100 mg | Standard Adult MDT Dose |
| Excipients | Q.S. | Magnesium Stearate / Starch |
*Pack Sizes: Blister packs of 10s (Box of 10×10), 28s (Calendar Pack), or Bulk HDPE Jars of 1000s (Program Packs).
Technical & Logistics Specifications
Critical data for Pharmaceutical Importers and Distributors.
| HS Code | 3004.90.99 (Other Medicaments) |
| Dosage Form | Tablet (Oral) |
| Packaging | PVC-Alu Blister or Alu-Alu Blister |
| Storage | Store below 25°C. Protect from light. |
| Certificates | WHO-GMP, COPP, Free Sale Certificate |
Manufacturing Authority
Marketed and Distributed by Healthy Inc from WHO-GMP & ISO 9001:2015 certified units.
- Cross-Contamination Control: Sulfones can cause severe allergic reactions (Stevens-Johnson Syndrome) in sensitized individuals. We manufacture Dapsone in segregated areas with dedicated air handling units (AHUs) to prevent cross-contamination with other general products.
- Particle Size: Dapsone has poor water solubility. We utilize micronized API to ensure adequate dissolution and bioavailability, which is critical for reaching effective skin and nerve tissue concentrations.
Therapeutic Indications (Human Use)
Indicated for the treatment of specific infectious and autoimmune diseases:
- Leprosy (Hansen’s Disease): A core component of the WHO Multi-Drug Therapy (MDT) regimen for both Paucibacillary and Multibacillary leprosy (combined with Rifampicin and Clofazimine).
- Dermatitis Herpetiformis (DH): The drug of choice for this blistering autoimmune skin condition associated with gluten sensitivity.
- Actinomycetoma: Treatment of certain fungal/bacterial infections of the foot.
Dosage & Administration
Recommended Dosage (Strictly as per Physician/WHO Guidelines):
- Route: Oral.
- Leprosy (Adults): 100 mg daily (usually for 6 to 12 months, depending on classification).
- Leprosy (Children): 1-2 mg/kg body weight daily.
- Dermatitis Herpetiformis: Start with 50 mg daily. Titrate up to 300 mg daily to control itching, then reduce to the lowest effective maintenance dose (often 25-50 mg daily).
- Timing: Can be taken with or without food. Taking with food may reduce gastric upset.
Safety Warnings:
- G6PD Deficiency (CRITICAL): Dapsone causes oxidative stress on red blood cells. In patients with Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency, it causes severe, potentially fatal Hemolysis (destruction of red blood cells). Screening is mandatory before starting therapy.
- Methemoglobinemia: Can convert hemoglobin to methemoglobin (which cannot carry oxygen), causing the patient to turn blue (cyanosis). Monitor oxygen saturation.
- Dapsone Hypersensitivity Syndrome: A rare, life-threatening reaction appearing after 4-6 weeks (fever, rash, hepatitis). Stop drug immediately.
- Sulfonamide Allergy: Cross-reactivity is possible; use with caution in patients allergic to sulfa drugs.
Global Export & Contract Manufacturing Services
Healthy Inc stands as a premier Pharmaceutical Exporter in India, dedicated to serving the needs of international Pharma Traders, Wholesalers, and Bulk Drug Distributors. As a verified Medicine Supplier in Mumbai, we offer flexible Third Party Manufacturing (Contract Manufacturing) services for OSD (Oral Solid Dosage) forms, allowing brands to launch high-quality generic medicines under their own label. Whether you are looking for a reliable Hospital Tender Supplier for government procurement in Africa or a B2B Pharma Marketplace partner for Latin America, our logistics network ensures timely delivery. We actively support Pharmaceutical Drop Shipping models and bulk indenting, ensuring that every Generic Medicine Wholesaler receives WHO-GMP certified products at competitive rates.
Commercial Inquiries
For hospital tenders, bulk export, or distributor pricing, please contact our export team.
WhatsApp/Call: +91 7710003340
Email: info@healthyinc.co.in