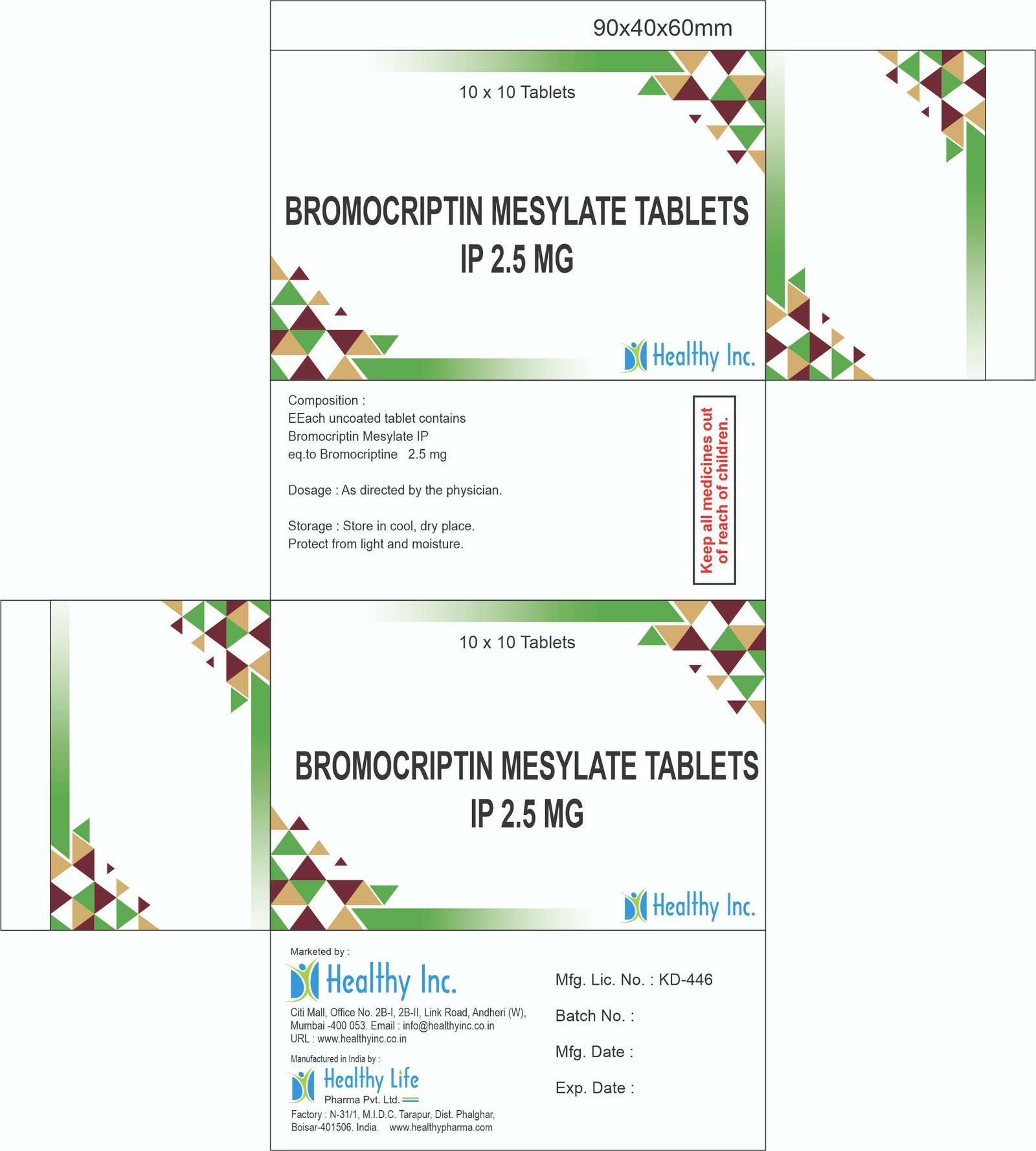
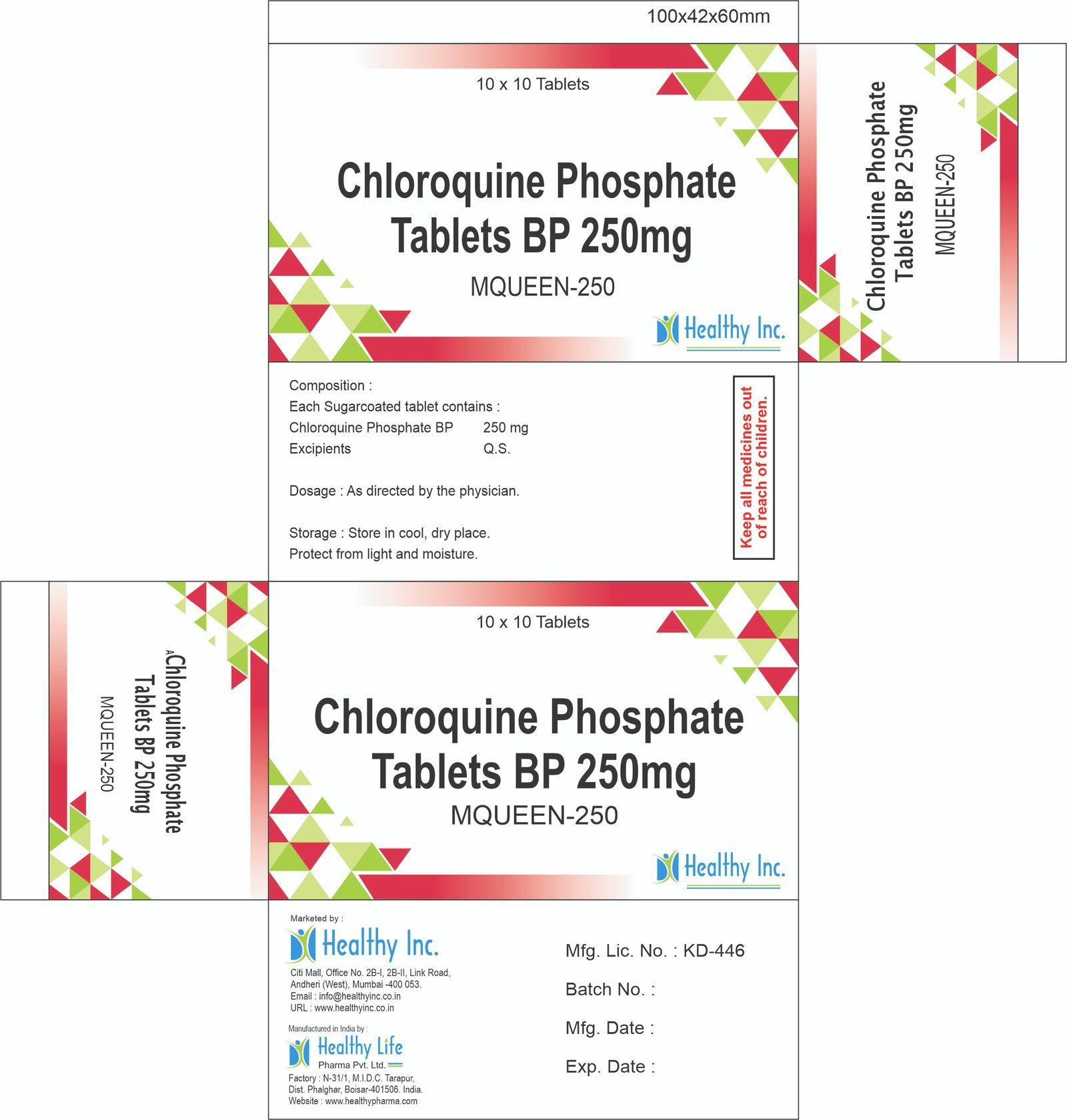
Description
Linezolid Injection 600 mg (IV Infusion)
Healthy Inc is a specialized global supplier and exporter of advanced anti-infective infusions. We provide high-purity Linezolid & Dextrose Anhydrous Injection, manufactured in our WHO–GMP certified facilities. This premium Oxazolidinone antibiotic is a “Last-Line Defense” exported to hospitals and critical care centers in LATAM, CIS, Africa, and Southeast Asia for the treatment of resistant superbugs like MRSA and VRE.
Product Overview
Linezolid is a synthetic antibiotic of the Oxazolidinone class. It works by binding to the bacterial 23S ribosomal RNA, preventing the formation of the 70S initiation complex—a unique mechanism that prevents cross-resistance with other antibiotics.
It is the “Gold Standard” for:
- MRSA Infections: Methicillin-Resistant Staphylococcus aureus (Skin, Soft Tissue, and Pneumonia).
- VRE Infections: Vancomycin-Resistant Enterococcus faecium.
- Nosocomial Pneumonia: Hospital-acquired pneumonia caused by resistant Gram-positive bacteria.
Product Composition & Strength
We supply this product as a Ready-to-Use IV Infusion in a Dextrose base.
| Active Ingredient | Strength (Per ml) | Total Content (Per Bag/Bottle) |
|---|---|---|
| Linezolid | 2 mg / ml | 600 mg / 300 ml |
| Dextrose Anhydrous | 50 mg / ml (5%) | Isotonic Vehicle |
*Supplied in 300ml FFS (Form-Fill-Seal) Plastic Bottles or IV Bags.
Technical & Logistics Specifications
Critical data for Pharmaceutical Importers and Hospital Tenders.
| HS Code | 3004.20.99 (Medicaments containing antibiotics) |
| Dosage Form | Liquid IV Infusion (Large Volume Parenteral) |
| Route of Administration | Intravenous (IV) Infusion |
| Packaging | 300ml FFS Bottle / Non-PVC Bag (with Overwrap) |
| Storage | Store below 25°C. Protect from light and freezing. |
| Certificates | WHO-GMP, COPP, FSC, CTD Dossier |
Manufacturing Authority
Manufactured at our WHO-GMP & ISO 9001:2015 certified unit.
- Sterility: Manufactured using BFS (Blow-Fill-Seal) technology for zero human contact and absolute sterility.
- Stability: Packaged with UV-protective overwrap as Linezolid is sensitive to light.
- Capacity: Dedicated IV fluid lines for high-volume hospital supply.
Therapeutic Indications
Indicated for the treatment of severe infections caused by susceptible Gram-positive bacteria:
- Vancomycin-resistant Enterococcus faecium infections.
- Nosocomial pneumonia (caused by S. aureus or S. pneumoniae).
- Complicated skin and skin structure infections (including Diabetic Foot Infections).
- Community-acquired pneumonia.
Dosage & Administration
Strict Medical Supervision Required (Hospital Use):
- Adults: 600 mg administered intravenously every 12 hours (Twice daily).
- Duration: Recommended treatment duration is 10 to 14 days (up to 28 days for VRE).
- Infusion Rate: Administer over a period of 30 to 120 minutes. Do not inject rapidly.
Warnings:
- MAO Inhibitors: Linezolid is a reversible inhibitor of monoamine oxidase. Avoid tyramine-rich foods and certain antidepressants.
- Myelosuppression: Monitor complete blood counts (CBC) weekly in patients receiving Linezolid, especially for treatments >2 weeks.
Commercial Inquiries
For hospital tenders, bulk export, or distributor pricing, please contact our export team.
WhatsApp/Call: +91 7710003340
Email: info@healthyinc.co.in