Description
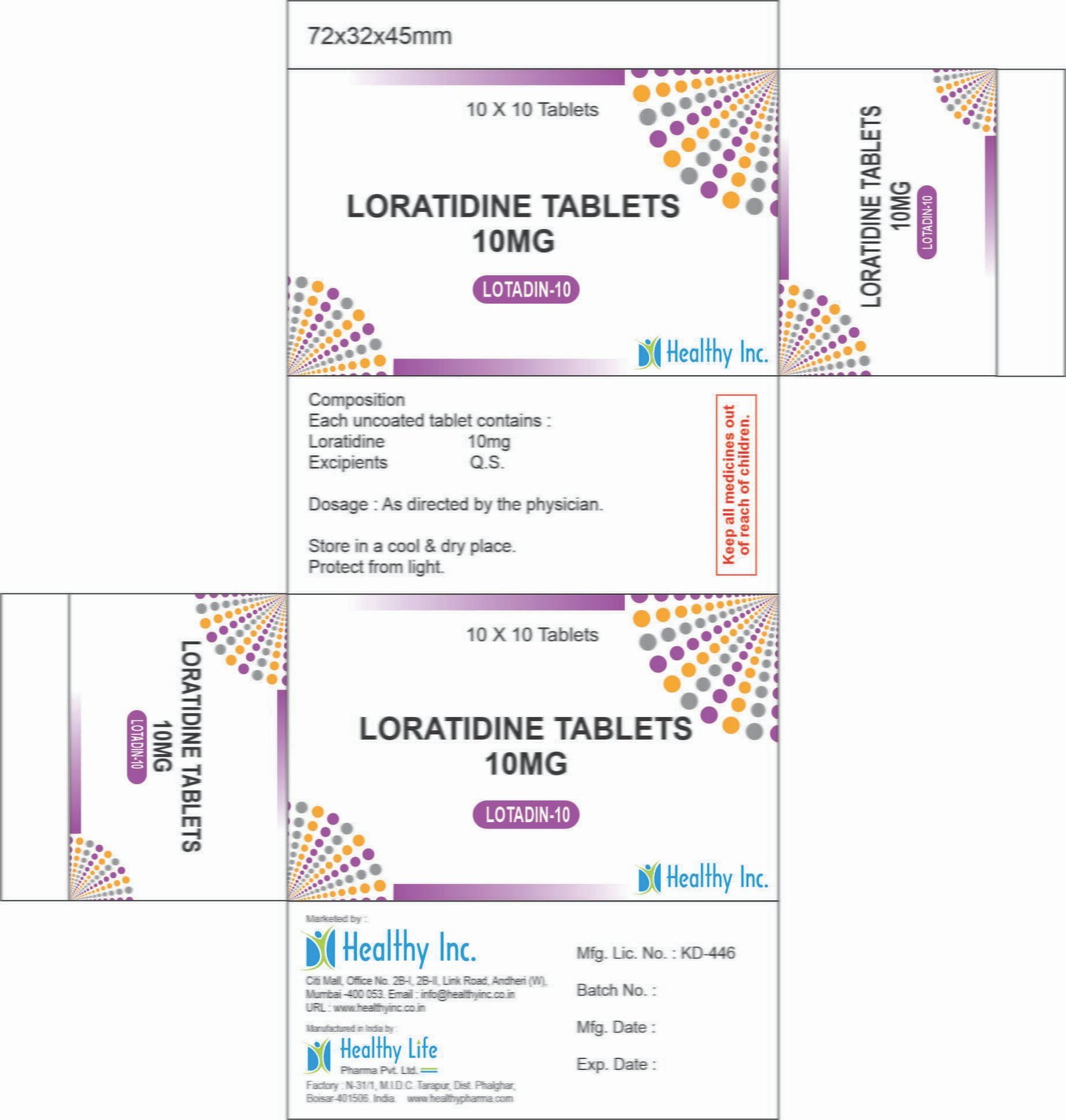
Loratadine Tablets (10 mg)
Healthy Inc is a premier global supplier of high-precision respiratory and anti-allergy therapies. We provide premium Loratadine Tablets, manufactured in WHO–GMP certified specialized solid dosage facilities. As a frontline treatment for allergic rhinitis and chronic urticaria, this product is a cornerstone export for community pharmacies, ENT specialists, and Ministry of Health (MOH) tenders in Southeast Asia, Africa, and the Middle East.
Product Overview
Loratadine is a potent, long-acting, second-generation tricyclic antihistamine. It is specifically engineered to provide 24-hour relief from allergy symptoms with a superior safety profile, characterized by its inability to cross the blood-brain barrier, thereby ensuring non-sedative performance.
The “Daytime Allergy” Specialist:
- Mechanism of Action: Loratadine is a selective peripheral H1-receptor antagonist. By blocking the action of histamine—the primary mediator of allergic reactions—it effectively prevents smooth muscle contraction, capillary permeability, and the “itch-flare” response without the drowsiness associated with first-generation antihistamines.
- Long-Duration Efficacy: With a half-life that supports once-daily dosing, Loratadine provides consistent symptomatic control throughout the day and night, improving patient compliance and quality of life.
- Metabolic Profile: It is metabolized by the liver into desloratadine, an even more potent active metabolite, ensuring a sustained pharmacological effect even after plasma levels of the parent drug begin to decline.
Technical & Manufacturing Specifications
Formulated for rapid disintegration and high chemical stability in all climates.
| Technical Metric | Specification Standard |
|---|---|
| Active Ingredient | Loratadine BP / USP / IP |
| Dosage Form | Immediate Release Tablets / Mouth Dissolving (MDT) |
| Available Strength | 10 mg |
| HS Code | 3004.90.99 (Antihistamines) / 2933.39.19 |
| Packaging | PVC-PVDC / Alu Blister (Moisture barrier protection) |
Manufacturing Authority
Marketed and Distributed by Healthy Inc from ISO 9001:2015 certified units.
- Non-Sedation Validation: Our formulation process ensures that the active ingredient remains pure and free from central-acting impurities, maintaining the clinical “non-drowsy” claim essential for active patients and drivers.
- Dissolution Optimization: We utilize specialized disintegrants to ensure the 10 mg dose is released rapidly within the gastric environment, leading to a quick Tmax and onset of symptom relief.
- Stability Validation: Validated for Zone IVb (40°C / 75% RH). Our tablets are engineered to withstand tropical humidity, ensuring the structural integrity and potency remain intact for a 36-month shelf life.
Therapeutic Indications & Clinical Symptoms
- Allergic Rhinitis: Relief of symptoms such as sneezing, nasal discharge (rhinorrhea), and itching of the nose and throat.
- Allergic Conjunctivitis: Management of watery, itchy, and red eyes.
- Chronic Idiopathic Urticaria: Treatment of hives, skin rashes, and localized itching.
- Seasonal Allergies: Effective control of “Hay Fever” symptoms during peak pollen seasons.
Dosage & Administration
- Adults and Children (>12 years): One 10 mg tablet once daily.
- Pediatrics (6-12 years): 10 mg once daily or as directed by a physician.
- Safety Note: While non-sedating for most, avoid alcohol as it may potentiate rare side effects. Use with caution in patients with severe hepatic impairment (initial dose of 10 mg every other day is recommended).
Global Export & Contract Manufacturing Services
Healthy Inc is a verified Pharmaceutical Exporter in Mumbai, catering to Retail Pharmacy Chains and Ministry of Health Tenders. We offer Third Party Manufacturing for Loratadine with full CTD Dossier and COPP support. Our logistics network ensures secure transit to Vietnam, Nigeria, UAE, and the Philippines, providing WHO-GMP quality at competitive B2B prices.
Commercial Inquiries
WhatsApp/Call: +91 7710003340
Email: info@healthyinc.co.in