Description
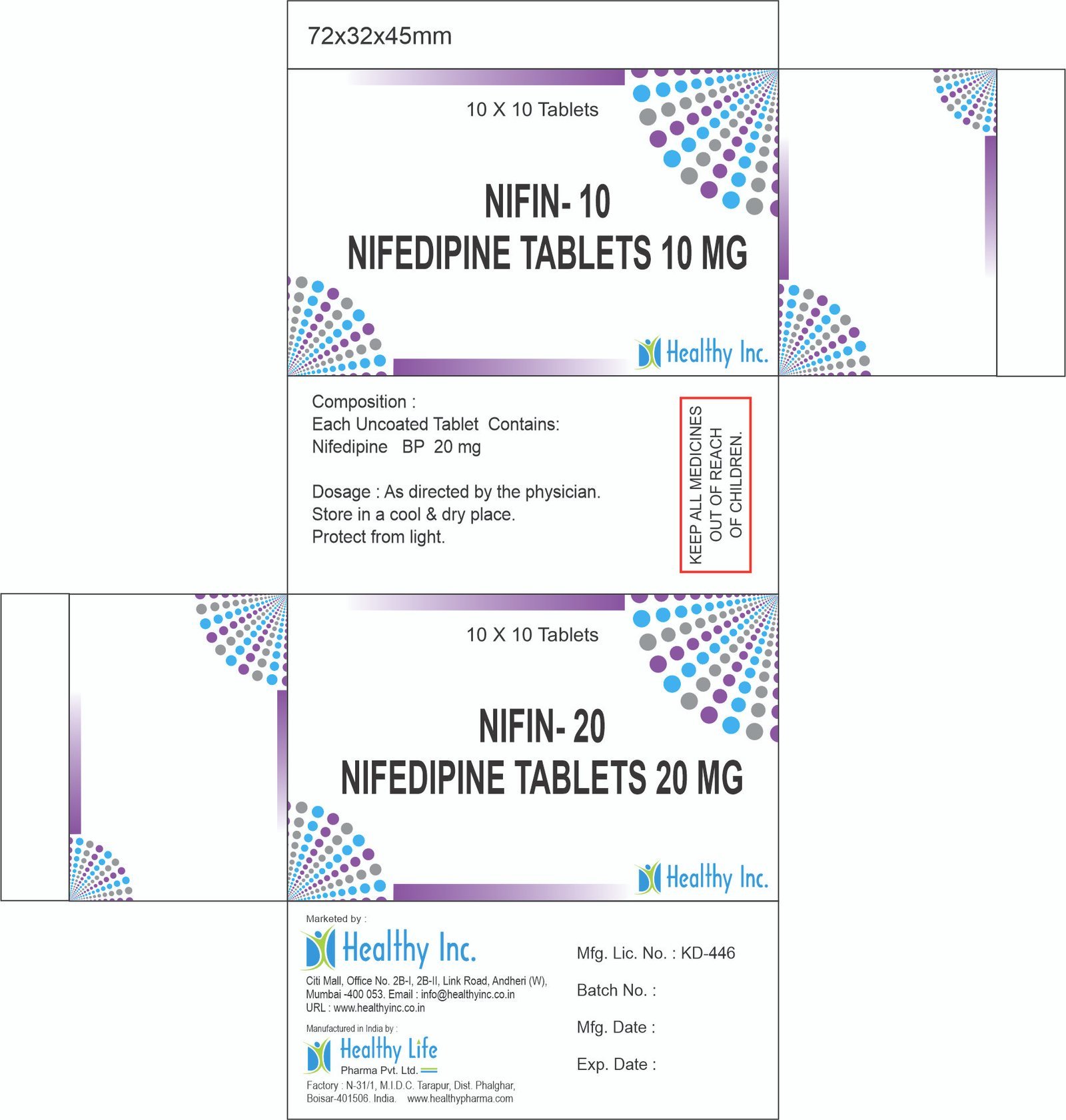
Nifedipine Tablets (10 mg / 20 mg / 30 mg / 60 mg)
Healthy Inc is a premier global supplier of high-stability cardiovascular therapies. We provide premium Nifedipine Tablets, available in immediate-release and Sustained-Release (SR/ER) formulations, manufactured in WHO–GMP certified facilities. As a critical vasodilator, this product is a cornerstone export for cardiology clinics, hypertension management programs, and Ministry of Health (MOH) tenders in Southeast Asia, Africa, and the Middle East.
Product Overview
Nifedipine is a potent dihydropyridine calcium channel blocker (CCB). It is specifically engineered to inhibit the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle, leading to profound systemic vasodilation.
The “Vascular Resistance” Specialist:
- Mechanism of Action: Nifedipine selectively inhibits the L-type calcium channels. By preventing calcium entry, it inhibits muscle contraction, resulting in the relaxation of coronary and peripheral arterial smooth muscle. This reduces total peripheral resistance and decreases blood pressure.
- Anti-Anginal Efficacy: By dilating the main coronary arteries and arterioles, Nifedipine increases myocardial oxygen delivery and prevents coronary artery spasms (Prinzmetal’s angina).
- Sustained-Release Excellence: While immediate-release Nifedipine is used for acute scenarios, our Sustained-Release (SR) technology provides 24-hour blood pressure control with a single dose, minimizing the risk of reflex tachycardia.
Technical & Manufacturing Specifications
Formulated for light-stability and controlled-release precision.
| Technical Metric | Specification Standard |
|---|---|
| Active Ingredient | Nifedipine BP / USP / IP |
| Dosage Form | Film-Coated / Sustained-Release (SR) Tablets |
| Available Strengths | 10 mg, 20 mg, 30 mg, 60 mg |
| HS Code | 3004.90.66 (Cardiovascular Drugs) / 2933.39.90 |
| Packaging | Alu-Alu Blister (Mandatory for light and moisture protection) |
Manufacturing Authority
Marketed and Distributed by Healthy Inc from ISO 9001:2015 certified units.
- Photosensitivity Management: Nifedipine is extremely sensitive to light. Our manufacturing process is conducted under sodium-vapor (yellow) lighting, and tablets are finished with an opaque film coating to prevent photodegradation.
- Osmotic Release Technology: Our 30mg and 60mg Extended-Release tablets utilize a laser-drilled osmotic pump system (GITS) to provide a constant release rate (zero-order kinetics), ensuring stable plasma levels.
- Micronization: Given its low water solubility, we utilize micronized Nifedipine API to ensure consistent absorption and bioequivalence with the innovator brand (Adalat/Procardia).
Therapeutic Indications & Clinical Symptoms
- Chronic Stable Angina: Long-term management of angina pectoris.
- Hypertension: Management of high blood pressure, either alone or in combination with other antihypertensives.
- Vasospastic (Prinzmetal’s) Angina: Treatment of confirmed or suspected coronary artery spasm.
- Raynaud’s Phenomenon: Off-label use for the management of digital vasospasm.
Dosage & Administration
- Standard Adult Dose: Usually 30 mg to 60 mg once daily for SR/ER formulations.
- Administration: Tablets must be swallowed whole; do not crush, chew, or break sustained-release tablets as this can lead to rapid drug release and toxicity.
- Safety Note: Avoid grapefruit juice during treatment as it significantly increases Nifedipine plasma concentrations.
Global Export & Contract Manufacturing Services
Healthy Inc is a verified Pharmaceutical Exporter in Mumbai, catering to Cardiology Hospital Chains and Ministry of Health Tenders. We offer Third Party Manufacturing for Nifedipine with full CTD Dossier and COPP support. Our logistics network ensures secure transit to Vietnam, Nigeria, UAE, and the Philippines, providing WHO-GMP quality at competitive B2B prices.
Commercial Inquiries
WhatsApp/Call: +91 7710003340
Email: info@healthyinc.co.in