Description
Sulfasalazine Delayed Release Tablets
Healthy Inc is a specialized global supplier and exporter of dual-action anti-inflammatory agents. We provide high-purity Sulfasalazine Delayed Release (Enteric Coated) Tablets, sourced from WHO–GMP certified solid dosage facilities. This “Gut & Joint” specialist is a top export to gastroenterology clinics, rheumatology departments, and chronic care formularies in Africa, LATAM, and Southeast Asia, serving as a foundational therapy for Ulcerative Colitis and Rheumatoid Arthritis.
Product Overview
This formulation contains Sulfasalazine, a prodrug consisting of 5-aminosalicylic acid (5-ASA) linked to sulfapyridine by an azo bond.
The “Site-Specific” Specialist:
- Colon-Targeted Delivery: The tablet is Enteric Coated (Delayed Release) to pass through the stomach intact. Once it reaches the colon, bacterial azoreductases cleave the azo bond, releasing the active anti-inflammatory agent (5-ASA) directly at the site of inflammation (the bowel wall).
- Dual Action:
- For Colitis: The 5-ASA component acts locally to reduce inflammation in the colon lining.
- For Arthritis: The Sulfapyridine component is absorbed systemically and acts as a Disease-Modifying Antirheumatic Drug (DMARD) to slow joint damage.
- Remission Maintenance: Highly effective in maintaining remission in mild to moderate Ulcerative Colitis, preventing flare-ups.
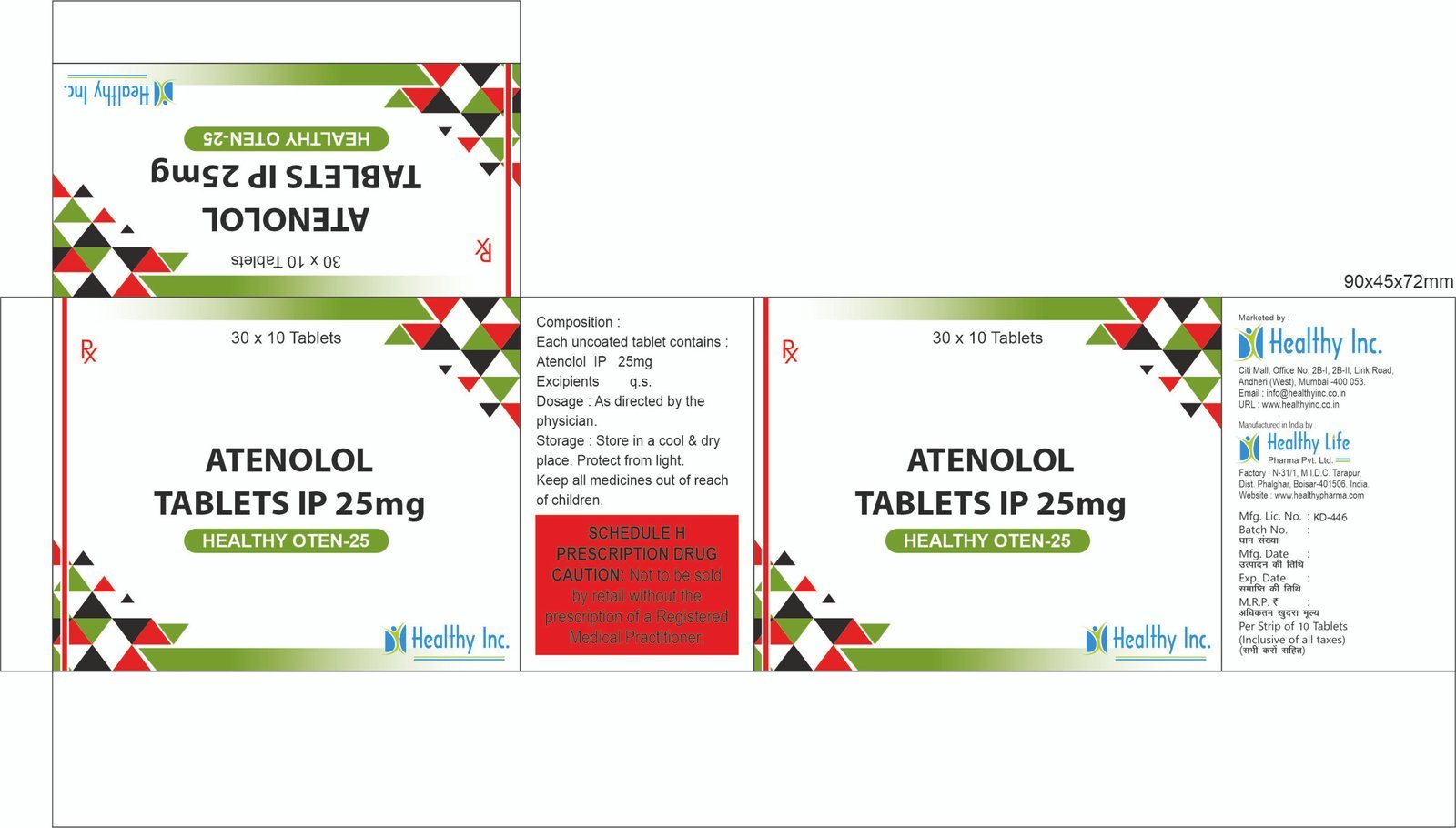
Product Composition & Strength
We supply this product as Enteric Coated (Gastro-Resistant) Tablets to prevent gastric irritation and ensure colonic delivery.
| Active Ingredient | Strength (Standard) | Form |
|---|---|---|
| Sulfasalazine USP/BP | 500 mg | Enteric Coated Tablet |
| Excipients | Q.S. | Cellulose Acetate Phthalate (Coating) |
*Pack Sizes: Blister packs of 10s (Box of 10×10) or Bulk Jars of 1000s (Hospital Packs).
Technical & Logistics Specifications
Critical data for Pharmaceutical Importers and Distributors.
| HS Code | 3004.90.99 (Other Medicaments) |
| Dosage Form | Delayed Release Tablet (Oral) |
| Packaging | Alu-Alu Blister or Amber HDPE Jar |
| Storage | Store below 25°C. Protect from Light and Moisture. |
| Certificates | WHO-GMP, COPP, Free Sale Certificate |
Manufacturing Authority
Marketed and Distributed by Healthy Inc from WHO-GMP & ISO 9001:2015 certified units.
- Coating Integrity: The enteric coating is critical. We perform rigorous dissolution testing (Stage 1: Acid Stage, Stage 2: Buffer Stage) to ensure no drug is released in the stomach, minimizing nausea and maximizing efficacy.
- Yellow Pigment: Sulfasalazine is naturally orange-yellow. Our manufacturing suites utilize specialized dust containment systems to handle this vibrant compound.
Therapeutic Indications (Human Use)
Indicated for inflammatory conditions:
- Ulcerative Colitis: Treatment of mild to moderate active UC and as adjunctive therapy in severe cases; prolongation of remission.
- Rheumatoid Arthritis (RA): Treatment of patients with RA who have responded inadequately to salicylates or other NSAIDs.
- Juvenile Idiopathic Arthritis: Management of polyarticular course juvenile rheumatoid arthritis.
Dosage & Administration
Recommended Dosage (Strictly as per Gastroenterologist/Rheumatologist):
- Route: Oral.
- Ulcerative Colitis (Adults): Initially 3 to 4 g daily in divided doses; maintenance dose 2 g daily.
- Rheumatoid Arthritis (Adults): Initially 500 mg to 1 g daily; typically increased to 2 g daily.
- Administration: Take after meals with a full glass of water. Do not crush or chew (destroys the enteric coating).
Safety Warnings:
- Sulfa Allergy: Strictly Contraindicated in patients with hypersensitivity to sulfonamides or salicylates (aspirin).
- Crystalluria: Patients must maintain high fluid intake to prevent kidney stones (crystals).
- Discoloration: Will turn urine and skin an orange-yellow color. This is harmless but can stain contact lenses.
- Male Fertility: Can cause temporary oligospermia (low sperm count) and infertility in men (reversible upon withdrawal).
Global Export & Contract Manufacturing Services
Healthy Inc stands as a premier Pharmaceutical Exporter in India, dedicated to serving the needs of international Pharma Traders, Wholesalers, and Bulk Drug Distributors. As a verified Medicine Supplier in Mumbai, we offer flexible Third Party Manufacturing (Contract Manufacturing) services for OSD (Oral Solid Dosage) forms, allowing brands to launch high-quality generic medicines under their own label. Whether you are looking for a reliable Hospital Tender Supplier for government procurement in Africa or a B2B Pharma Marketplace partner for Latin America, our logistics network ensures timely delivery. We actively support Pharmaceutical Drop Shipping models and bulk indenting, ensuring that every Generic Medicine Wholesaler receives WHO-GMP certified products at competitive rates.
Commercial Inquiries
For hospital tenders, bulk export, or distributor pricing, please contact our export team.
WhatsApp/Call: +91 7710003340
Email: info@healthyinc.co.in