Showing all 4 results
-
Amlodipine besylate with Losartan potassium Tablets
FreeAmlodipine Tablets IP 5 mg
Each Uncoated tablet contains :
Amlodipine Besylate IP
Eq to Amlodipine 5 mgAmlodipine Tablets IP 10 mg
Each Uncoated Tablet Contains :
Amlodipine Besylate IP
Eq To Amlodipine 10 mgAmlodipine Besylate Tablets IP 2.5 mg
Each uncoated tablet contains :
Amlodipine Besylate IP
Eq. To Amlodipine 2.5 mgAmlodipine Besylate Tablets IP 2.5 mg
Each Film Coated Tablet Contains:
Amlodipine Besylate IP
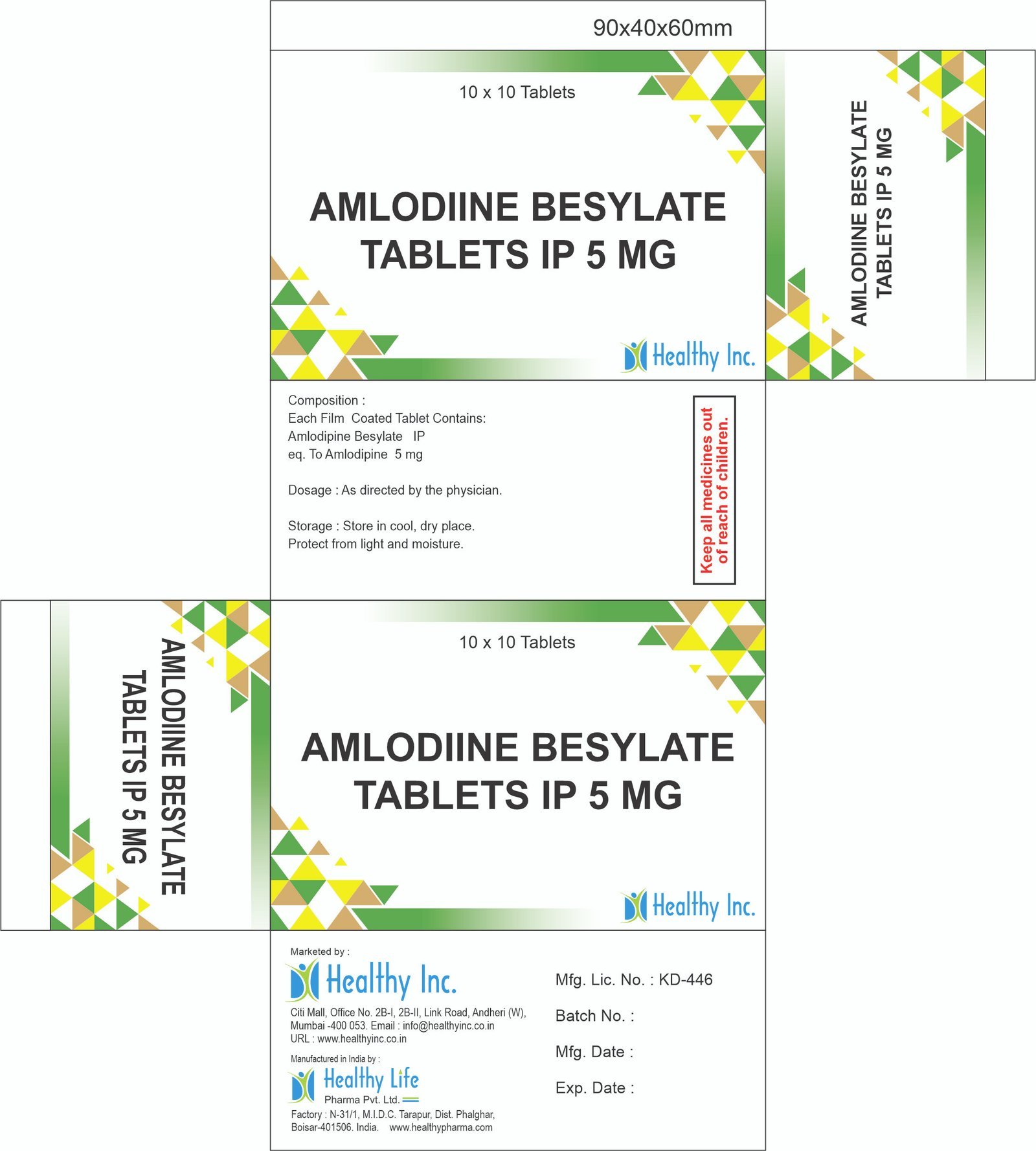
eq. To Amlodipine 2.5 mgAmlodipine Besylate Tablets IP 5 mg
Each Film Coated Tablet Contains:
Amlodipine Besylate IP
eq. To Amlodipine 5 mgAMLODIPINE & ENALAPRIL MALEATE TABLET
Each Film Coated Tablet Contains :
Amlodipine Besylate
Eq. To Amlodipine 5 mg
Enalapril Maleate 5 mg
Excipients q.sAMLODIPINE & LISINOPRIL TABLET
Each Film Coated Tablet Contains :
Amlodipine Besylate
Eq. To Amlodipine 5 mg
Lisinopril dihydrate
Eq. To Lisinopril 5 mg
Excipients q.sTRIPPLEACE AMLODIPINE -10 / AMLODIPINE
TABLETS 10 MG
Each uncoated tablet contains:
Amlodipine Besilate BP
Equivalent to Amlodipine 10 mg
Excipients Q.S.Usage: – Treat high blood pressure
Category: – Hypertensive / Cardiac Drugs
Therapeutic category: – hypertensive, Cardiovascular Agent,
-
Amlodipine With Enalapril maleate Tablets
FreeAmlodipine Tablets IP 5 mg
Each Uncoated tablet contains :
Amlodipine Besylate IP
Eq to Amlodipine 5 mgAmlodipine Tablets IP 10 mg
Each Uncoated Tablet Contains :
Amlodipine Besylate IP
Eq To Amlodipine 10 mgAmlodipine Besylate Tablets IP 2.5 mg
Each uncoated tablet contains :
Amlodipine Besylate IP
Eq. To Amlodipine 2.5 mgAmlodipine Besylate Tablets IP 2.5 mg
Each Film Coated Tablet Contains:
Amlodipine Besylate IP
eq. To Amlodipine 2.5 mgAmlodipine Besylate Tablets IP 5 mg
Each Film Coated Tablet Contains:
Amlodipine Besylate IP
eq. To Amlodipine 5 mgAMLODIPINE & ENALAPRIL MALEATE TABLET
Each Film Coated Tablet Contains :
Amlodipine Besylate
Eq. To Amlodipine 5 mg
Enalapril Maleate 5 mg
Excipients q.sAMLODIPINE & LISINOPRIL TABLET
Each Film Coated Tablet Contains :
Amlodipine Besylate
Eq. To Amlodipine 5 mg
Lisinopril dihydrate
Eq. To Lisinopril 5 mg
Excipients q.sTRIPPLEACE AMLODIPINE -10 / AMLODIPINE
TABLETS 10 MG
Each uncoated tablet contains:
Amlodipine Besilate BP
Equivalent to Amlodipine 10 mg
Excipients Q.S.Usage: – Treat high blood pressure
Category: – Hypertensive / Cardiac Drugs
Therapeutic category: – hypertensive, Cardiovascular Agent,
-
Atenolol with Chlorthalidone tablets.
Freetenolol Tablets IP 25 mg Each Uncoated Tablet
contains :- Atenolol IP 25 mg
Excipients – QSAtenolol Tablets IP 50 mg
Each Uncoated Tablet contains :
Atenolol IP 50 mg
Excipients q.sOtenol 50
Atenolol Tablets BP
Each Uncoated tablet contains :
Atenolol BP 50 mgOtenol
Atenolol Tablets BP
Each Uncoated tablet contains :
Atenolol BP 100 mgUsage: – Treat high blood pressure and irregular heartbeat
Category: – Hypertensive Cardiac drugs
Therapeutic category: – Cardiovascular Agent,Anti hypertensive
-
Glimepiride with metformin Tablet
FreeGlimepiride Tablets IP 1 mg
Each Filmcoated tablet contains :
Glimepiride IP 1 mgGlimepiride Tablets IP 2 mg
Each Filmcoated tablet contains :
Glimepiride IP 2 mgGlimepiride Tablets 1 mg
Each Filmcoated tablet contains :
Glimepiride BP 1 mgGlimepiride Tablets 2 mg
Each Filmcoated tablet contains :
Glimepiride BP 2 mgGlimepiride Tablets 4 mg
Each Film Coated Tablet Contains:
Glimepiride BP 4 mgSMEGLIP -1 / GLIMEPIRIDE TABLETS BP 1 MG Each Filmcoated Tablet Contains: Glimepiride BP 1 mg
Excipients – QSSMEGLIP -2 / GLIMEPIRIDE TABLETS BP 2 MG Each Filmcoated Tablets Contains: Glimepiride BP 2 mg
Excipients – QSGLIMEPIRIDE,PIOGLITAZONE & METMIN HYDROCHLORIDE SR TABLET
Each Sustained Release Tablet Contains :
Glimepiride 2 mg
Pioglitazone 15 mg
Metmin Hydrochloride 500 mg
Excipients q.sUsage: – Type 2 diabetes is a condition where the body doesn’t make enough insulin, or the insulin that it makes doesn’t work properly
Category: – Anti Diabetic drugs
Therapeutic category: – Anti Diabetic
Pcd pharma franchise:-
Pcd marketing:-
Manufacturer: – Healthy Life Pharma Pvt Ltd
Exporter: – Healthy Inc
Supplier: – Healthy Life Pharma Pvt Ltd
Healthy Inc
Distributor: – Healthy Life Pharma Pvt Ltd
Healthy Inc
Seller: – Healthy Life Pharma Pvt Ltd
Healthy Inc